The smallest repeating unit of a crystal of common salt is a cube (called a unit cell)
Question:
The smallest repeating unit of a crystal of common salt is a cube (called a unit cell) with an edge length of 0.563 nm.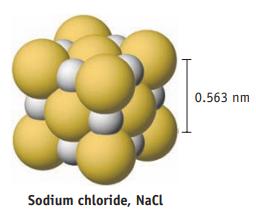
(a) What is the volume of this cube in cubic nanometers? In cubic centimeters?
(b) The density of NaCl is 2.17 g/cm3. What is the mass of this smallest repeating unit (“unit cell”)?
(c) Each repeating unit is composed of four NaCl units. What is the mass of one NaCl formula unit?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





