The spectrum shown here is for aspirin. The vertical axis is the amount of light absorbed, and
Question:
The spectrum shown here is for aspirin. The vertical axis is the amount of light absorbed, and the horizontal axis is the wavelength of incident light (in nm). 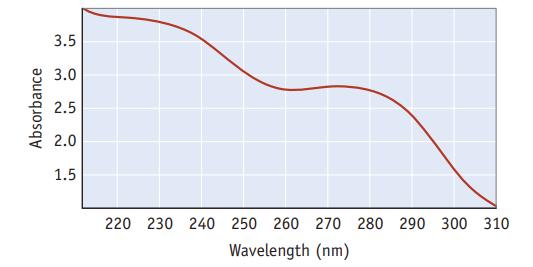
What is the frequency of light with a wavelength of 278 nm? What is the energy of one mole of photons with λ = 278 nm? What region of the electromagnetic spectrum is covered by the spectrum above? Knowing that aspirin only absorbs light in the region depicted by this spectrum, what is the color of aspirin?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:





