Use standard reduction potentials (Appendix M) for the half-reactions AgBr(s) + e Ag(s) + Br
Question:
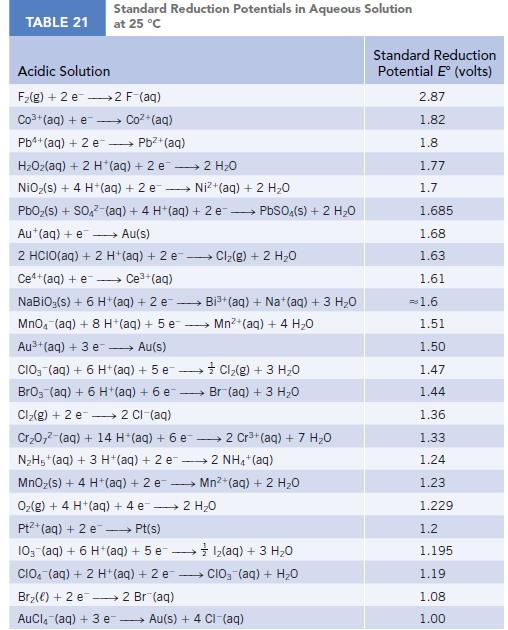
Use standard reduction potentials (Appendix M) for the half-reactions AgBr(s) + e− → Ag(s) + Br−(aq) and Ag+(aq) + e− → Ag(s) to calculate the value of Ksp for AgBr.
Data given in Appendix M
Transcribed Image Text:
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+ (aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+ (aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+ (aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO3(aq) + 6 H+ (aq) + 5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂ (s) + 4 H+ (aq) + 2 e O₂(g) + 4 H+ (aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4H₂O Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)
2303 RT E log Ksp nF nFE log Ksp 2303 RT 1x9...View the full answer

Answered By

Arun kumar
made more than four thousand assignments
5.00+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
The standard reduction potentials of the following half-reactions are given in Appendix E: (a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest...
-
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2 e Zn (s) + 4 OH (aq) and Zn 2+ (aq) + 2e Zn(s) to calculate the value of K formation for the...
-
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following reactions at 298 K. Data given in Appendix M (a) CIO3(aq) + 5 Cl(aq) + 6 H+ (aq) 3 Cl(g) + 3 HO(l) (b)...
-
A rectangular pontoon 10m long 7m broad & 2.5m deep weights 686.7KN. It carries on its upper deck an empty boiler of 5m diameter weighing 588.6 KN. The centre of gravity of the boiler and pontoon are...
-
Koko Chocolate Company makes dark chocolate and light chocolate. Both products require cocoa and sugar. The following planning information has been made available: Koko Chocolate does not expect...
-
On December 31, 2022, Waters Company prepared an income statement and balance sheet, but failed to take into account three adjusting entries. The balance sheet showed total assets $150,000, total...
-
True or false: RMS stands for relative mean squared.
-
Albers Company acquires an 80% interest in Barker Company on January 1, 2011, for $850,000. The following determination and distribution of excess schedule is prepared at the time of purchase: Albers...
-
A currenty situation I'm in is buying new furniture and appliances for my new home, its a constant struggle balancing what I want and how much it costs. A linear way of making this decision is by...
-
Diagram the apparatus used to electrolyze molten NaCl. Identify the anode and the cathode. Trace the movement of electrons through the external circuit and the movement of ions in the electrolysis...
-
Calculate r G and the equilibrium constant for the following reactions. (a) Zn+ (aq) + Ni(s) Zn(s) + Ni+ (aq) (b) Cu(s) + 2 Ag+ (aq) = Cu+ (aq) + 2 Ag(s)
-
The income statement of Marx Co. for the month of July shows net income of $1,500 based on Service Revenue $5,500; Wages Expense $2,300; Supplies Expense $900, and Utilities Expense $800. In...
-
Complete a written analysis of leadership within the film (use the topics you have covered in your readings, assignments. (100 POINTS) This assignment provides the opportunity to synthesize your...
-
Check the articles that talk about (possible shift) in Boeing's Operations Strategy. You are more than welcome to find more in the same alignment. Your main job is to try to compare and contrast. How...
-
Gender schema indicates that we are influenced by society's ideas about what it means to be a male or female in the culture(s) in which we are a part of. Thus, it can be thought of as an organized...
-
Adult children who take care of their aging parent(s) should not do so alone, the adult child needs support as well. The article attached discusses how siblings should work together to care for their...
-
Technological progress results in firms experiencing an ongoing state of change. Using a change model of your choice , describe how you would advise M&Co to manage the ongoing changes associated with...
-
Refer to Exercise 16.12. Estimate with 95% confidence the mean price of 60,000 sq.ft. apartment buildings.
-
Question 6.10 Current and deferred tax worksheets and tax entries From the hip Ltd?s statement of profit or loss for the year ended 30 June 2007 and extracts from its statements of financial position...
-
A hockey puck slides along a rough, icy surface. It has an initial velocity of 35 m/s and slides to a stop after traveling a distance of 95 m. Find the coefficient of kinetic friction between the...
-
A rock is dropped from a very tall tower. If it takes 4.5 s for the rock to reach the ground, what is the height of the tower?
-
A baseball is hit directly upward with an initial speed of 45 m/s. Find the velocity of the ball when it is at a height of 40 m. Is there one correct answer for v or two? Explain why.
-
A stock will pay a dividend of $7.07 in one year. If your discount rate is 0.11 per year, and the growth rate in dividends is a constant 0.02 per year, what is current stock price?
-
A firm plans to issue new shares of preferred stock to fund a new project. The new preferred stock shares will offer an annual dividend of $6 and will be sold to investors at a price of $100. The...
-
Date: January 1, 2021 Platte Valley Paul is the owner of a diversified farm business located in northeastern Colorado. He got his start in the family operation about ten years ago. By using equipment...

Study smarter with the SolutionInn App


