Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH) 4 ] 2 (aq) + 2
Question:
Use the standard reduction potentials (Appendix M) for the half-reactions [Zn(OH)4]2−(aq) + 2 e− → Zn (s) + 4 OH−(aq) and Zn2+(aq) + 2e− → Zn(s) to calculate the value of Kformation for the complex ion [Zn(OH)4]2−.
Data given in Appendix M
Transcribed Image Text:
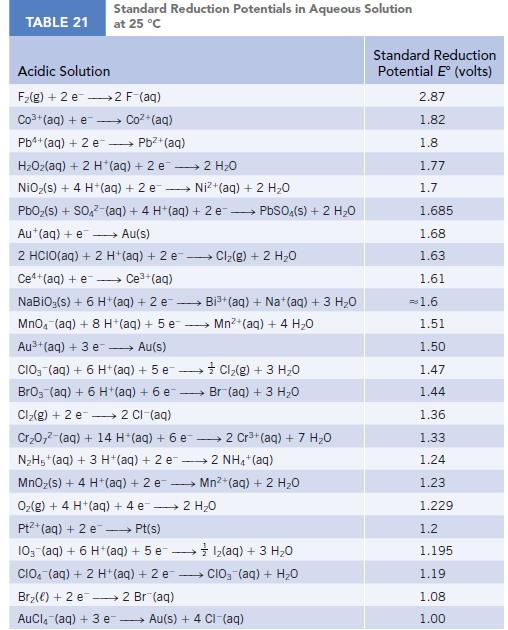
TABLE 21 Standard Reduction Potentials in Aqueous Solution at 25 °C Acidic Solution F₂(g) + 2 e 2 F-(aq) Co3+ (aq) + e Coz+(aq) Pb4+ (aq) + 2 e - Pb²+ (aq) HzOz(aq) + 2 H*(aq) +2e → 2H2O NiO₂(s) + 4 H+ (aq) + 2 e→→→→→→ Ni+(aq) + 2 HO PbO₂ (s) + SO4² (aq) + 4 H+ (aq) + 2e → PbSO4(s) + 2 H₂O Au+ (aq) + e→→→→→ Au(s) 2 HCIO(aq) + 2 H+ (aq) + 2 e-- - Ce+(aq) + e→→→→ Ce³+ (aq) NaBiO;(s) + 6 H+ (aq) + 2 e- → MnO4 (aq) + 8 H+ (aq) + 5 e Au³+ (aq) + 3 e→→→→→ Au(s) CIO3(aq) + 6 H+ (aq) + 5 e→→→→→→ BrO3 (aq) + 6 H+ (aq) + 6 e- Cl₂(g) + 2 e 2 Cl-(aq) Cr₂0,² (aq) + 14 H*(aq) + 6 e 2 Cr³+ (aq) + 7 H₂O N₂H5+ (aq) + 3 H+ (aq) + 2 e2 NH4+ (aq) MnO₂ (s) + 4 H+ (aq) + 2 e O₂(g) + 4 H+ (aq) + 4 e 2 H₂O Pt+ (aq) + 2 e →→→→→ Pt(s) 10- (aq) + 6 H+ (aq) + 5 e1₂(aq) + 3 H₂O CIO (aq) + 2 H+ (aq) + 2 e CIO₂ (aq) + H₂O Br₂() +2 e 2 Br(aq) AuCl(aq) + 3 e → Cl₂(g) + 2 H₂O → Bi³+ (aq) + Na+ (aq) + 3 H₂O Mn²+ (aq) + 4H₂O Cl₂(g) + 3 H₂O Br (aq) + 3 H₂O Mn²+ (aq) + 2 H₂O Au(s) + 4 CI-(aq) Standard Reduction Potential E (volts) 2.87 1.82 1.8 1.77 1.7 1.685 1.68 1.63 1.61 = 1.6 1.51 1.50 1.47 1.44 1.36 1.33 1.24 1.23 1.229 1.2 1.195 1.19 1.08 1.00
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 80% (5 reviews)

Answered By

Hassan Imtiaz
The following are details of my Professional Experience. Responsibilities Eight years of demanding teaching experience in the field of finance and business studies at Master’s Level. Completion of the given tasks within given time with quality and efficiency. Marketing professional with practical experience in and solid understanding of a diverse range of management applications, including market analysis, sales and marketing, team building and quality assurance. I have excellent skills to approach deal and sustain corporate clients / customers by demonstrating not only extraordinary communication and interpersonal skills but also high caliber presentation, negotiation and closing skills. Manage and follow up the day-to-day activities. Manage and co-ordinate the inventories. Fulfillment of all the tasks assigned.
The following are details of my Areas of Effectiveness. Finance 1. Corporate Finance 2. Advanced Corporate Finance 3. Management of Financial Institutions 4. International Financial Management 5. Investments 6. Fixed Income 7. Real Estate Investment 8. Entrepreneurial Finance 9. Derivatives 10. Alternative Investments 11. Portfolio Management 12. Financial Statement Analysis And Reporting (US GAAP & IFRS) 13. International Financial Markets 14. Public Finance 15. Personal finance 16. Real estate 17. Financial Planning Quantitative Analysis 1. Time Value Of Money 2. Statistics 3. Probability Distribution 4. Business Statistics 5. Statistical Theory and Methods Economics 1. Principles of Economics 2. Economic Theory 3. Microeconomic Principles 4. Macroeconomic Principles 5. International Monetary Economics 6. Money and Banking 7. Financial Economics 8. Population Economics 9. Behavioral Economics International Business 1. Ethics 2. Business Ethics 3. An introduction to business studies 4. Organization & Management 5. Legal Environment of Business 6. Information Systems in Organizations 7. Operations Management 8. Global Business Policies 9. Industrial Organization 10. Business Strategy 11. Information Management and Technology 12. Company Structure and Organizational Management Accounting & Auditing 1. Financial Accounting 2. Managerial Accounting 3. Accounting for strategy implementation 4. Financial accounting 5. Introduction to bookkeeping and accounting Marketing 1. Marketing Management 2. Professional Development Strategies 3. Business Communications 4. Business planning 5. Commerce & Technology Human resource management 1. General Management 2. Conflict management 3. Leadership 4. Organizational Leadership 5. Supply Chain Management 6. Law 7. Corporate Strategy Creative Writing 1. Analytical Reading & Writing Other Expertise 1. Risk Management 2. Entrepreneurship 3. Management science 4. Organizational behavior 5. Project management 6. Financial Analysis, Research & Companies Valuation 7. And any kind of Excel Queries
4.80+
150+ Reviews
230+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Use the standard reduction potentials (Appendix M) for the half-reactions [AuCl 4 ] (aq) + 3 e Au(s) + 4 Cl (aq) and Au 3+ (aq) + 3 e Au(s) to calculate the value of K formation for the complex...
-
Use the standard reduction potentials (Appendix M) for the half-reactions Hg 2 Cl 2 (s) + 2e 2 Hg() + 2 Cl (aq) and Hg 2 2+ (aq) + 2 e 2 Hg() to calculate the value of K sp for Hg 2 Cl 2 . Data...
-
The standard reduction potentials of the following half-reactions are given in Appendix E: (a) Determine which combination of these half-cell reactions leads to the cell reaction with the largest...
-
Cummings Corp. has three business segmentsthe Plumbing Parts division, Small Tools division, and Design Printing division. Carol Jenkins (Cummings' CFO) and William Samuels (head of Production) would...
-
Gulf Coast Resins Company processes a base chemical into plastic. Standard costs and actual costs for direct materials, direct labor, and factory overhead incurred for the manufacture of 2,600 units...
-
The unadjusted trial balance for Sierra Corp. is shown in Illustration 4.5 . Instead of the adjusting entries shown in the text at October 31, assume the following adjustment data. 1. Supplies on...
-
True or false: Zero to peak and peak readings are the same thing.
-
A gear reduction unit uses the countershaft shown in the figure. Gear A receives power from another gear with the transmitted force FA applied at the 20_ pressure angle as shown. The power is...
-
When examining criminal justice agencies, the most important characteristic to employees was the feeling that the job was important and yielded a feeling of accomplishment. Job satisfaction differs...
-
Diagram the apparatus used to electrolyze molten NaCl. Identify the anode and the cathode. Trace the movement of electrons through the external circuit and the movement of ions in the electrolysis...
-
Use standard reduction potentials (Appendix M) for the half-reactions AgBr(s) + e Ag(s) + Br (aq) and Ag + (aq) + e Ag(s) to calculate the value of K sp for AgBr. Data given in Appendix M TABLE...
-
If countries were brands, what metrics do you think these brands monitor: the U.S., China, Japan, Germany, Brazil? Are these brand managers watching the right indicators?
-
How do legal frameworks, policy interventions, and institutional reforms contribute to shaping the direction and pace of social change, particularly in addressing systemic injustices and advancing...
-
How do social movements and collective action strategies catalyze and sustain processes of social change, and what are the key tactics and mobilization strategies employed by activists to effect...
-
Jed Clampett is expanding his family-run beer distributorship into Georgia or Tennessee. His parents began the business many years ago and now three generations of Clampetts work in the family...
-
1. (5 points) What does "deepcopy" do? (the answer: "it makes a deep copy" will not earn any points) 2. (10 points) Referring to the following sample python line of code: def Number Three(Apples,...
-
Kyrie Irving made headlines during the 2021 NBA Season because he was unable to play basketball for any home game because of the NYC vaccination mandate. However, he was allowed to play all away...
-
Refer to Exercise 16.13. Predict with 99% confidence the price of a 1999 24-ft. Sea Ray cruiser with 400 hours of engine use.
-
Research an article from an online source, such as The Economist, Wall Street Journal, Journal of Economic Perspectives, American Journal of Agricultural Economics, or another academic journal. The...
-
A ball is thrown upward with a speed of 35 m/s from the edge of a cliff of height h - 15 m (similar to Fig. P3.22). (a) What is the speed of the ball when it passes by the cliff on its way down to...
-
Jeff Gordon (a race car driver) discovers that he can accelerate at 4.0 m/s 2 without spinning his tires, but if he tries to accelerate more rapidly, he always burns rubber. (a) Find the coefficient...
-
Anti lock brakes. A car travels at 65 mi/h when the brakes are suddenly applied. Consider how the tires of a moving car come in contact with the road. When the car goes into a skid (with wheels...
-
A project requires an initial investment in equipment of $90,000 and then requires an investment in working capital of $10,000 at the beginning (t = 0). The project is expected to produce sales...
-
Use the given Information to draw a right triangle. Use trigonometric ratios and a calculator to find the unknown sides and angles. Round off sides and angles to the same number of decimal places as...
-
A firm's bonds currently have 20 years to maturity. These bonds have a 9.25% annual coupon, sell at a price of $1,075, and have a par value of $1,000. If the firm's tax rate is 40% , what is the...

Study smarter with the SolutionInn App


