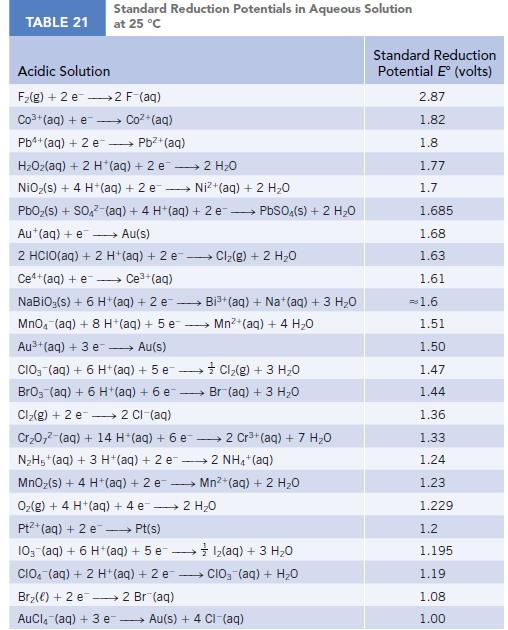
Use the table of standard reduction potentials (Appendix M) to calculate r G for the following
Question:
Use the table of standard reduction potentials (Appendix M) to calculate ΔrG° for the following reactions at 298 K.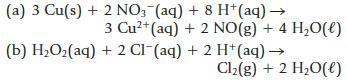
Data given in Appendix M
Transcribed Image Text:
(a) 3 Cu(s) + 2NO3(aq) + 8 H+ (aq) → 3 Cu²+ (aq) + 2 NO(g) + 4 H₂O(l) (b) H₂O₂(aq) + 2 Cl(aq) + 2 H+ (aq) → Cl₂(g) + 2 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a AG 1 FE n6 b n2 F96500 E ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Fe(s) + Ni2+(aq) Fe2+ (aq) + Ni(s) (b) Co(s) + 2 H+...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Cu(s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag(s) (b) 3 Ce4+...
-
Compare the following two regressions: i. Y, =B + B, X, + e, ii. Y, =B + B(2X) + e, Equation i. is exactly the regression we've been working with thus far, so all the formulas we've derived thus far...
-
Refer to E 29 and respond to the following requirements. Data in E 2-9 Prepare the necessary adjusting entries on December 31, 2024, for the Microchip Company for each of the following situations....
-
Wal-Mart intended to build a new store in Florence, South Carolina. Because the store would have a parking lot, it needed a permit that determined how stormwater runoff would be handled. The parking...
-
Kate contracts with Bernardo to transport Bernardos goods to his stores. If this contract is discharged as most contracts are, it will be discharged by a. performance. b. agreement. c. operation of...
-
A project has been selected for implementation. The net cash flow (NCF) profile associated with the project is shown below. MARR is 10 percent/year. a. What is the internal rate of return of this...
-
ASW Publishing, Inc., a small publisher of college textbooks, must make a decision regarding which books to publish next year. The books under consideration are listed in the following table, along...
-
What is the interest earned on $350 invested 4 years at a 5% simple interest?If I put $1500 into my savings account and earned $180 of interest at 4% simple interest, how long was my money in the...
-
Write balanced equations for the following reduction half-reactions involving organic compounds. (a) HCOH CHO (b) C6H5COH C6HCH3 (c) CH,CH,CHO (d) CH3OH CH CHCHCHOH (acid solution) (acid solution)...
-
What is marketing, and what is the goal of the marketing process?
-
The Mistry Company has the following sales, inventory, and purchases during the fiscal year ended December 31, 2023. Mistry Company uses a perpetual inventory system. Required 1. Calculate the dollar...
-
On October 31, 2015, Pete's Print Shop purchased a copy machine for $27,600. Pete's Print Shop expects the machine to last for three years and to have a residual value of $1,500. Compute depreciation...
-
Fifteen years ago, Hailey invested $5,000 and locked in an annual interest rate of 6 percent for 30 years ( ending 15 years from now ). Aidan can make a 15-year investment today and lock in an...
-
1. Sales Revenue: a. The price is $10 and the volume sold was 345,000 units. What is sales revenue? b. Sales revenue is $55,110 and the price is $11. What is the volume sold? c. The volume sold was...
-
Information about the ending inventories of Charleston Chair Company, who applies the LIFO method, is shown below: Current Normal Replacement Selling Cost of Profit Year Cost Cost Price Completion...
-
A pension fund manager is holding a 10-year 10% coupon bond in the funds portfolio, and the interest rate is currently 10%. What loss would the fund be exposed to if the interest rate rises to 11%...
-
An All-Pro defensive lineman is in contract negotiations. The team has offered the following salary structure: Time Salary 0 .......... $ 8,500,000 1 .......... 3,900,000 2 .......... 4,600,000 3...
-
Q1) What is the a3 Value Q2) What is the a7 Value Q3) What is the a4 Value Q4) What is the b3 Value Q5) What is the b2 Value Q6) What is the sign of 2nd constraint? A pastry chef at a bakery wants to...
-
A superposition wave function can be expanded in the eigenfunctions of the operator corresponding to an observable to be measured. In analogy to rolling a single die, each of the infinite number of...
-
If a system is in an eigenstate of the operator of interest, the wave function of the system can be determined. Explain this assertion. How could you know that the system is in an eigenstate of the...
-
If the wave function for a system is a superposition wave function, the wave function of the system cannot be determined. Explain this assertion.
-
Which do you find most interesting: the creation of messages, the nature of message characteristics, or the interpretation and response to messages? To what extent are you interests shaped by your...
-
What does it mean to decolonise? How is this framework important in your media practice?
-
What is HIPPA? Why is it relevant to the practice of professional counseling? What does it require?

Study smarter with the SolutionInn App


