What volume of 0.125 M oxalic acid, H 2 C 2 O 4 , is required to
Question:
What volume of 0.125 M oxalic acid, H2C2O4, is required to react with 35.2 mL of 0.546 M NaOH?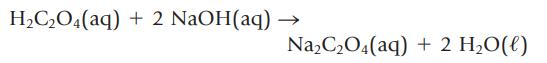
Transcribed Image Text:
H₂C₂O4(aq) + 2 NaOH(aq) Na₂C₂O4(aq) + 2 H₂O(l)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
To determine the volume of 0125 M oxalic acid H2C2O4 required to react with 352 mL of 0546 M N...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Question Posted:
Students also viewed these Sciences questions
-
Marnie wants to save up $250,000 to pay cash for a home purchase 15 years from now. If her investment can carn 6.1% compounded monthly and she intends to grow each payment by 0.25%, what will be her...
-
1. What mass of H2 should be produced by the reaction of Al with 75.0 mL of 2.95M HCl? 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2 (g). ln the lab, 0.15g H2 was collected. What is the % yield of the...
-
For Reaction 1-7, how many milliliters of 0.165 0 M KMnO4 are needed to react with 108.0 mL of 0.1650 M oxalic acid? How many milliliters of 0.1650 M oxalic acid are required to react with 108.0 mL...
-
Landry State University, a public university located in Louisiana, has a June 30 fiscal year. On July 20 of the current year, Landry State University receives $2,125,000 in payments from the U.S....
-
Describe the concept of Six Sigma quality. Why is such a high quality level important?
-
We hear the expression "taking a quantum leap" to describe large changes. Is the expression appropriate? Defend your answer.
-
A fluid with a density of \(2000 \mathrm{~kg} / \mathrm{m}^{3}\) flows steadily between two flat plates as shown in Fig. P6.23. The bottom plate is fixed and the top one moves at a constant speed in...
-
This problem requires the use of ACL software, which is included in the CD attached to the text. Information about installing and using ACL and solving this problem can be found in Appendix, pages...
-
Dollar-Value LIFO On January 1, 2018, Sato Company adopted the dollar-value LIFO method of inventory costing. Sato's ending inventory records appear as follows: Year Current Cost Index 2018 $31,600...
-
What volume of 0.812 M HCl, in milliliters, is required to titrate 1.45 g of NaOH to the equivalence point? NaOH(aq) + HCl(aq) HO(l) + NaCl(aq)
-
What volume of 0.750 M Pb(NO 3 ) 2 , in milliliters, is required to react completely with 1.00 L of 2.25 M NaCl solution? The balanced equation is Pb(NO3)2(aq) + 2 NaCl(aq) PbCl(s) + 2 NaNO3(aq)
-
Circle K was once one of the largest convenience store chains in the United States. Circle K separated its products into two major categories: gasoline and merchandise (Twinkies, beef jerky, soda...
-
Determine whether each procedure described below is an internal control strength or weakness. Weakness or Strength Internal Control Principle 1. Large amounts of cash are kept in a desk drawer to pay...
-
Suppose that the one - year interest rate is 4 . 0 percent in the United States; the spot exchange rate is $ 1 . 2 5 / ; and the one - year forward exchange rate is $ 1 . 1 6 / . What must the one -...
-
In what ways is a corporation recognized as a person? List the ways and explain each.
-
Generativity represents a major unifying theme in middle-aged adults life stories. Generative adults develop a way of thinking about the self that fosters a caring, compassionate approach to others....
-
Let b=log3 2, a = log, 2, prove that 2a = b. 700
-
When is bad debts expense recorded when using the allowance method?
-
Consider a closed, rigid tank with a volume of 0.8L, filled with cold water initially at 27C. The tank is filled such that there are no voids (air pockets) within. The initial pressure within the...
-
The Chalk Point, Maryland, generating station supplies electrical power to the Washington, D.C., area. Units 1 and 2 have a gross generating capacity of 710. MW (megawatt). The steam pressure is 25 ...
-
An electrical motor is used to operate a Carnot refrigerator with an interior temperature of 0.00C. Liquid water at 0.00C is placed into the refrigerator and transformed to ice at 0.00C. If the room...
-
Below are two hypothetical compounds. a) Which compound would you expect to hold greater promise as a potential antihistamine? Explain your choice. b) Do you expect the compound you chose (in part a)...
-
Which is better to disseminate information to stakeholders in an educational setting, bottom - up or top - down approach?
-
When Democratic members of Congress were considering the Affordable Care Act, many were divided on the bill. Some favored it and others opposed it . What would be the role of the whip in this...
-
When dealing with a knowledgeable, specialized audience, what is one good idea to include in your presentation?

Study smarter with the SolutionInn App


