You can analyze for a copper compound in water using an instrument called a spectrophotometer. [A spectrophotometer
Question:
You can analyze for a copper compound in water using an instrument called a spectrophotometer. [A spectrophotometer is a scientific instrument that measures the amount of light (of a given wavelength) that is absorbed by the solution.] The amount of light absorbed at a given wavelength of light (A) depends directly on the mass of compound per liter of solution. To calibrate the spectrophotometer, you collect the following data 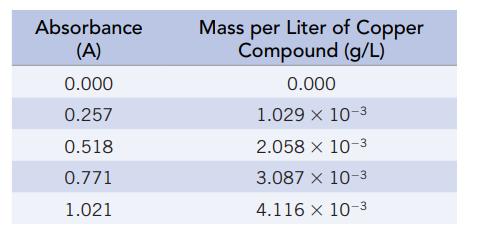
Plot the absorbance (A) against the mass of copper compound per liter (g/L), and find the slope (m) and intercept (b) (assuming that A is y and the amount in solution is x in the equation for a straight line, y = mx + b). What is the mass of copper compound in the solution in g/L and mg/mL when the absorbance is 0.635?
Step by Step Answer:

Chemistry And Chemical Reactivity
ISBN: 9780357001172
10th Edition
Authors: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel




