A 0.2500-g sample of an AlZn alloy reacts with HCl to form hydrogen gas: The hydrogen produced
Question:
A 0.2500-g sample of an Al–Zn alloy reacts with HCl to form hydrogen gas:
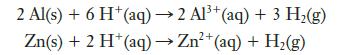
The hydrogen produced has a volume of 0.147 L at 25°C and 755 mm Hg. What is the percentage of zinc in the alloy?
Transcribed Image Text:
2 Al(s) + 6 H+ (aq) → 2 Al³+ (aq) + 3 H₂(g) Zn(s) + 2 H+ (aq) → Zn²+ (aq) + H₂(g)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (6 reviews)

Answered By

Muhammad Umair
I have done job as Embedded System Engineer for just four months but after it i have decided to open my own lab and to work on projects that i can launch my own product in market. I work on different softwares like Proteus, Mikroc to program Embedded Systems. My basic work is on Embedded Systems. I have skills in Autocad, Proteus, C++, C programming and i love to share these skills to other to enhance my knowledge too.
3.50+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
A 6.11-g sample of a Cu-Zn alloy reacts with HCl acid to produce hydrogen gas. If the hydrogen gas has a volume of 1.26 L at 22C and 728 mmHg, what is the percent of Zn in the alloy? (Cu does not...
-
Rank the following alkenes in order of their stability, Put the most stable first I II III IV O A. IV > || > II>| O B. IV > || >|> II OC. II > I| >1> IV O D.I> I| > III > IV
-
(A) The reaction of aluminum with hydrochloric acid produces hydrogen gas. The balanced chemical equation for the reaction is given below. 2 Al(s) + 6 HCl(aq) 2 AlCl 3 (aq) + 3 H 2 (g) If 35.5 mL of...
-
Need help with the incorrectanswer. I have tried 6,750, 81,000, and 0, but all areincorrect. Exercise 4-23 (Algorithmic) (LO. 4) Casper and Cecile divorced in 2018. As part of the divorce settlement,...
-
Fred Barone is describing budgetary control. What steps should be included in Freds description?
-
Many contractors work on custom jobs that require a job order costing system. Required Access the Website AMSI.com and click on Construction Management Software, and then on STARBUILDER. Prepare a...
-
Juliette Shulof Furs (JSF) was a New York corporation that had been in the fur-dealing business for 15 years. George Shulof, an officer of JSF, attended two auctions conducted by Finnish Fur Sales...
-
The following is a linear programming model for analyzing the product mix of Maxines hat Company, which produces three hat styles: Maximize: $7x1+ $8x2 + $6x3 = Z Subject to: 2x1 + 4x2 + 2x3 ¤...
-
Various tools are available for developing and managing an auditing project plan and associated elements of an audit. Which tool is designed to enable an auditor to track audit deficiencies and areas...
-
You work in a semiconductor production plant that relies on several chlorofluorocarbons in its manufacturing process. One day, you find an unlabeled gas cylinder, and you are assigned to figure out...
-
A number of compounds containing the heavier noble gases, and especially xenon, have been prepared. One of these is xenon hexafluoride (XeF 6 ), which can be prepared by heating a mixture of xenon...
-
(a) Find the escape velocity (that is, the velocity above which a particle will escape to r = ) for a particle on a spherical planet of radius R and mass M. What is the numerical value for the earth?...
-
Solve 6 1 (8- -5 (8 9x) ax
-
Assume that the MPC is 0.85 and investment spending rises by $100 million. How much consumption spending will this generate in the second round of spending?
-
Discuss what is meant by "business" for purposes of taxation. Describe the scope of the VAT and the Percentage Tax. Note: Philippine Taxation
-
What is the difference between a subsidy granted to import-competing firms and an export subsidy? What is the purpose of each of these subsidies? Who are the beneficiaries (winners) under each of...
-
What three major aspects of the Texas constitution have changed through amendment? How did Texans influence the process to make changes?
-
Austin, a single individual with a salary of $100,000, incurred and paid the following expenses during the year: Medical expenses ...................$5,000 Alimony ......................24,000...
-
The first law of thermodynamics is sometimes whimsically stated as, You cant get something for nothing, and the second law as, You cant even break even. Explain how these statements could be...
-
Compare the electrostatic potential maps for cycloheptatrienone and cyclopentadienone. Both of these maps were created using the same color scale so they can be compared. Notice the difference...
-
What is the advantage of a differential scanning calorimeter over a bomb calorimeter in determining the enthalpy of fusion of a series of samples?
-
You wish to measure the heat of solution of NaCl in water. Would the calorimetric technique of choice be at constant pressure or constant volume? Why?
-
On January 5, 2023, Lieux purchased a bond paying interest at 6% for $30,000. On September 30, 2023, Lieux gave the bond to Greer. The bond pays $1,800 interest on December 31. Lieux and Greer are...
-
Xerox's internal benchmarking strategy has paid off by examining the operations of its various country divisions. For example, Xerox Europe, a $6 billion subsidiary of Xerox Corp., formed teams to...
-
Identify the data visualization chart type. A stacked bar chart shows the sales revenue for products A to E in percentage values. The y axis ranges from 0% to 100.00% in increments of 10%.

Study smarter with the SolutionInn App


