According to an article published in 1966 in the Journal of Chemical Education, xenon reacts photochemically with
Question:
According to an article published in 1966 in the Journal of Chemical Education, xenon reacts photochemically with fluorine at room temperature (298 K) to produce XeF2. A portion of a table showing ratios of gases in the reaction vessel for four experiments is reproduced below. (Assume that atmospheric pressure is exactly 1 atm.) The reaction is as shown:
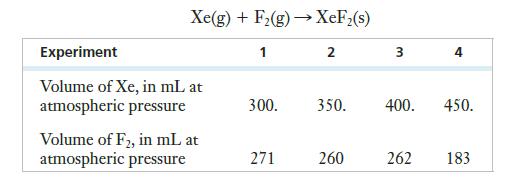
(a) Draw the Lewis structure of XeF2 and predict the molecule’s shape.
(b) Calculate the partial pressure of each gas in Experiment 3 if the amounts shown are combined such that the total pressure remains 1 atm.
(c) Calculate the theoretical yield of XeF2 in Experiment 3.
(d) After the reactants were introduced, the bulb was placed in sunlight. XeF2 began crystallizing on the inner surface of the bulb about 24 hours later. Compare the rate of this reaction to the rate of precipitation of CaF2 from solutions of Ca2+(aq) and F–(aq) in the laboratory.
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





