Calculate the equilibrium constant for the following reactions using data from the standard reduction potential tables. (a)
Question:
Calculate the equilibrium constant for the following reactions using data from the standard reduction potential tables.
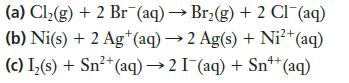
Transcribed Image Text:
(a) Cl₂(g) + 2 Br (aq) → Br₂(g) + 2 Cl¯(aq) (b) Ni(s) + 2 Ag+ (aq) →2 Ag(s) + Ni²+ (aq) (c) I₂(s) + Sn²+ (aq) → 2 I (aq) + Sn++ (aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
To calculate the equilibrium constant for the following reactions using data from the standard reduction potential tableswe can use the Nernst equatio...View the full answer

Answered By

Mwangi Clement
I am a tried and tested custom essay writer with over five years of excellent essay writing. In my years as a custom essay writer, I have completed more than 2,000 custom essays in a diverse set of subjects. When you order essays from me, you are working with one of the best paper writers on the web. One of the most common questions I get from customers is: “can you write my essay?” Upon hearing that request, my goal is to provide the best essays and overall essay help available on the web. I have worked on papers in subjects such as Nursing and Healthcare, English Literature, Sociology, Philosophy, Psychology, Education, Religious Studies, Business, Biological Sciences, Communications and Media, Physical Sciences, Marketing and many others. In these fields, my specialties lie in crafting professional standard custom writings. These include, but are not limited to: research papers, coursework, assignments, term papers, capstone papers, reviews, summaries, critiques, proofreading and editing, and any other college essays.
My extensive custom writings experience has equipped me with a set of skills, research abilities and a broad knowledge base that allows me to navigate diverse paper requirements while keeping my promise of quality. Furthermore, I have also garnered excellent mastery of paper formatting, grammar, and other relevant elements. When a customer asks me to write their essay, I will do my best to provide the best essay writing service possible. I have satisfactorily offered my essay writing services for High School, Diploma, Bachelors, Masters and Ph.D. clients.
I believe quality, affordability, flexibility, and punctuality are the principal reasons as to why I have risen among the best writers on this platform. I deliver 100% original papers that pass all plagiarism check tests (Turnitin, Copyscape, etc.). My rates for all papers are relatively affordable to ensure my clients get quality essay writing services at reasonable prices.
4.50+
5+ Reviews
14+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Fe(s) + Ni2+(aq) Fe2+ (aq) + Ni(s) (b) Co(s) + 2 H+...
-
Using the standard reduction potentials listed in Appendix E, calculate the equilibrium constant for each of the following reactions at 298 K: (a) Cu(s) + 2 Ag+ (aq) Cu2+ (aq) + 2 Ag(s) (b) 3 Ce4+...
-
From the following reduction potentials (a) Calculate the equilibrium constant for .I2 (aq) + I- I-3 (b) Calculate the equilibrium constant for I2 (aq) + I- I-3 (c) Calculate the solubility (g/L) of...
-
For Questions consider the S-N curve provided below for this same material and situation. Stress & (MPa) 400 300 B. 175 MPa C. 200 MPa 200 100 10 C. 350 MPa D. 400 MPa E. It will never fail P-0.99...
-
The word persuasion turns some people off. What negative connotations can it have?
-
List the costs that would likely be included in each of the following marginal-cost calculations. 1. The marginal cost of one additional passenger on an American Airlines flight. 2. The marginal cost...
-
Walt Disney reports the following information for its two Parks and Resorts divisions. Assume Walt Disney uses a balanced scorecard and sets a target of 85% occupancy in its resorts. Using Exhibit...
-
Barker Company has a single product called a Zet. The company normally produces and sells 80,000 Zets each year at a selling price of $40 per unit. The company's unit costs at this level of activity...
-
Discuss at least five of the formatting decisions you would have to make when typing a table. Compare the decisions you provided to those of two of your classmates. Do you agree or disagree with the...
-
Some calculators cannot display results of an antilog calculation if the power of 10 is greater than 99. This shortcoming can come into play for determining equilibrium constants of redox reactions,...
-
Use the standard reduction potentials for the reactions: to calculate the value of Ksp for silver chloride at 298 K. How does your answer compare with the value listed in Table 12.4? Table 12.4...
-
Sap travels in phloem as a result of _________. A. Evaporation of sugar water from the leaves; B. The phloem pump; C. A countercurrent to xylem flow; D. Differences in water pressure in a phloem tube...
-
Albert House, current workers' compensation carrier has provided Albert House, with a forecasted amount for 2024's workers' compensation claims. The total projected losses for 2024 are almost twice...
-
Based on historical data, the Hospital of St. Jacques wants to have 7 bags of B positive blood to every 2 bags of B Negative, furthermore, they want to have 3 bags of B negative blood for every 1 bag...
-
Balance Sheet Accounts of Athens Corporation Account Accumulated Depreciation Accounts Payable Accounts Receivable Cash Common Stock Inventory Long-Term Debt Plant, Property & Equipment Retained...
-
You want to be able to withdraw $25,000 each year for 15 years. Your account earns 7% interest. How much do you need in your account at the beginning?
-
Starware Software was founded last year to develop software for gaming applications. The founder initially invested $800,000 and received 9 million shares of stock. Starware now needs to raise a...
-
Repeat Prob. 124 for the following: (a) = F/wt, where F = 1 kN, w = 25 mm, and t = 5 mm. (b) I = bh3/12, where b = 10 mm and h = 25 mm. (c) I = d4/64, where d = 25.4 mm. (d) = 16 T/d3, where T = 25...
-
In the current year, the City of Omaha donates land worth $500,000 to Ace Corporation to induce it to locate in Omaha and create an estimated 2,000 jobs for its citizens. a. How much income, if any,...
-
The assembly consists of two 10-mm diameter red brass C83400 copper rods AB and CD, a 15-mm diameter 304 stainless steel rod EF, and a rigid bar G. If the horizontal displacement of end F of rod EF...
-
The assembly consists of two 10-mm diameter red brass C83400 copper rods AB and CD, a 15-mm diameter 304 stainless steel rod EF, and a rigid bar G. If P = 5 kN, determine the horizontal displacement...
-
The truss is made of three A-36 steel members, each having a cross-sectional area of 400 mm 2 . Determine the magnitude P required to displace the roller to the right 0.2 mm.
-
4. Sodium sulphide (Na2S) is added to pure water at a concentration of 103 M. Using an algebraic solution, determine the pH and the concentrations of all species at equilibrium, taking into account....
-
Task 7. From the six tuning rules, select the two norm response plots (i.e., the second plots) that you believe to be the most different. What insights do you believe these can provide on the results...
-
An iron square hollow tube with outer side of 50mm, inner side 40mm and 6m in length is subjected to a tensile load of 180kN. Young's Modulus of steel is 200kN/mm, calculate : a. the stress in the...

Study smarter with the SolutionInn App


