Chromium can be detected in atomic absorption spectroscopy by monitoring the absorbance of UV light at a
Question:
Chromium can be detected in atomic absorption spectroscopy by monitoring the absorbance of UV light at a wavelength of 357.8 nm. What is the energy of a photon of this light?
Strategy We know the connection between photon energy and wavelength, which is given by Equation 6.3. Again, care with units requires conversion from nanometers to meters.
Equation 6.3.
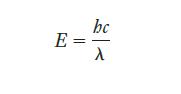
Transcribed Image Text:
E = bc λ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
Analyze Your Answer The result is a very small number But we should realize that P...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
A helium-neon laser emits laser light at a wavelength of 632.8 nm and a power of 2.3 mW. At what rate are photons emitted by this device?
-
The laser used in cornea surgery to treat corneal disease is the excimer laser, which emits ultraviolet light at a wavelength of 193 nm in air. The index of refraction of the cornea is 1.376. What...
-
The active medium in a particular laser that generates laser light at a wavelength of 694 nm is 6.00 cm long and 1.00 cm in diameter. (a) Treat the medium as an optical resonance cavity analogous to...
-
Tin - Can, Inc. Aircraft ( TCAI ) R&D Project Management Problem Your group is hired to help TCAI Project Manager to solve the following problem. Using the activity time estimates and activity...
-
Thome Company uses a flexible budget for manufacturing overhead based on direct labor hours. Variable manufacturing overhead costs per direct labor hour are as follows. Indirect labor ........ $1.00...
-
Clare manages a piano store. Her utility function is given by Utility = w 100 Where w is the total of all monetary payments to her and 100 represents the cost to her of the effort of running the...
-
What are the two purposes of taking a deposition?
-
RAR Corporations accounting records include the following items, listed in no particular order, at December 31, 2012: Income tax of 30% applies to all items. Requirement 1. Prepare RARs income...
-
Design a slider-crank mechanism so that the displacement of the slider is proportional to the square of the crank rotation in the interval 45 <0 135. Use three point Chebyshev spacing.
-
Unlike XRF, AAS cannot be used for nondestructive testing. Explain why not.
-
Consider a room that is 14 ft 20 ft with an 8-ft ceiling. (a) How many molecules of air are present in this room at 20C and 750 torr? (b) If a pollutant is present at 2.3 ppm, how many pollutant...
-
One student is selected from the student body of your college. Define the following events: Mthe student selected is male, Fthe student selected is female, Sthe student selected is registered for...
-
What is the angular diameter (in arcseconds) of a comet nucleus that has a radius of 15.0 km and is located at a distance of 3.00 Astronomical Units.
-
A 2.85010 3 kg car is parked on a steep hill inclined at 21.0 o . What is the force of friction acting on the car? The coefficients of friction between the tires and the road are k = 0.400 and s =...
-
You need to build a spring scale for weighing (actually building it is not necessary, this is theoretical exercise.) It will be helpful to sketch out a design. You need a scale capable of weighing...
-
A physics 11 student decides to test the theory of gravity by riding his bike off a building with horizontal roof. However, being a smart student he checks his landing area and discovers that there...
-
Ayayai Itzek manufactures and sells homemade wine, and he wants to develop a standard cost per gallon. The following are required for production of a 50-gallon batch. 2,910 ounces of grape...
-
Give an example of employee fraud and identify reasons why it may occur.
-
Read the case study Richter: Information Technology at Hungarys Largest Pharma and answer the following question: How does the organization ensure the accuracy of the data it stores?
-
Compound A has molecular formula C 9 H 8 O 2 and exhibits a strong signal at 1740 cm -1 in its IR spectrum. Treatment with two equivalents of LAH followed by water gives the following diol. Identify...
-
Rank each set of compounds in order of increasing acidity: a. b.
-
Malonic acid has two acidic protons: The pKa of the first proton (pK 1 ) is measured to be 2.8, while the pK a of the second proton (pK 2 ) is measured to be 5.7. (a) Explain why the first proton is...
-
On November 1, 2022, Sandhill Inc. adopted a stock option plan that granted options to key executives to purchase 32,300 shares of the company's common shares. The options were granted on January 2,...
-
In its income statement for the year ended December 31, 2025, Blossom Inc. reported the following condensed data. Operating expenses $710,000 Interest revenue $23,000 Cost of goods sold 1,246,000...
-
22. Assume that the following current asset and current liability accounts apply to the Harden Company for the years 2021 and 2022 (note: the ??? in the table is a figure that you must determine)....

Study smarter with the SolutionInn App


