Consider the apparatus shown, in which two 1.00-L bulbs are initially separated from one another by a
Question:
Consider the apparatus shown, in which two 1.00-L bulbs are initially separated from one another by a closed valve. One bulb is filled with 500 torr of sulfur dioxide and the other with 400 torr of oxygen, and the entire apparatus is at an initial temperature of 27°C.
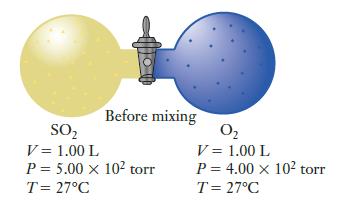
The valve separating the bulbs is opened so that the gases mix, the entire container is heated, and the two gases react to produce the maximum possible amount of SO3. If the container is at a final temperature of 600 K when the reaction reaches completion, what is the total pressure?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





