Question: Consider a container like that shown in Figure 20.12, with n 1 moles of a monatomic gas on one side and n 2 moles of
Consider a container like that shown in Figure 20.12, with n1 moles of a monatomic gas on one side and n2moles of a diatomic gas on the other. The monatomic gas has initial temperature T1i.
Figure 20.12
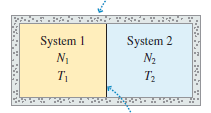 The diatomic gas has initial temperature T2i.
The diatomic gas has initial temperature T2i.
a. Show that the equilibrium thermal energies are
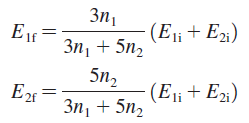
b. Show that the equilibrium temperature is
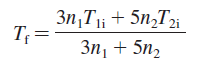
c. 2.0 g of helium at an initial temperature of 300 K interacts thermally with 8.0 g of oxygen at an initial temperature of 600 K. What is the final temperature? How much heat energy is transferred, and in which direction?
System 1 N1 System 2 N2 T1 T2 1 ( + Ex) li , + 5n, 5n2 (E+ E2) E2F , + 5n>
Step by Step Solution
3.41 Rating (167 Votes )
There are 3 Steps involved in it
Solve a The thermal energy of a monatomic gas of n 1 moles at an initial temperature T 1i i... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778ba0fac_693702.pdf
180 KBs PDF File
1442_6054778ba0fac_693702.docx
120 KBs Word File


