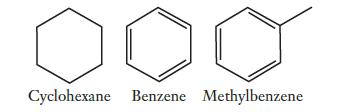
Consider the hydrocarbons whose structures are shown below. Which of these molecules would be planar, meaning that
Question:
Consider the hydrocarbons whose structures are shown below. Which of these molecules would be planar, meaning that all of the atoms must lie in the same plane? Explain your answer in terms of orbital hybridizations.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





