Question: Consider the structure shown below for N 2 O 3 as well as any other important resonance structures. (a) What is the expected ONO bond
Consider the structure shown below for N2O3 as well as any other important resonance structures.
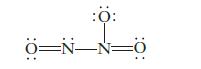
(a) What is the expected O—N—O bond angle in this structure?
(b) The N2O3 molecule contains N—O bonds of two different lengths. How many shorter N—O bonds would be present?
:0: =N- 0=N_N=0
Step by Step Solution
3.41 Rating (179 Votes )
There are 3 Steps involved in it
a 120 b There will be one shorter bond The d... View full answer

Get step-by-step solutions from verified subject matter experts


