Question: Given the equilibria: determine the equilibrium expression for the sum of the two reactions. Strategy Write the equilibrium expressions for the two reactions. Multiply them
Given the equilibria:
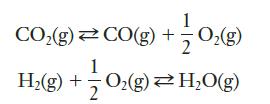
determine the equilibrium expression for the sum of the two reactions.
Strategy
Write the equilibrium expressions for the two reactions. Multiply them to obtain the equilibrium expression for the sum of the two reactions. Check your answer by adding the two reactions and comparing the sum to the equilibrium expression you determined.
CO(g) H(g) + 2 CO(g) + O(g) +1/1/20 0(g) O(g) HO(g)
Step by Step Solution
3.29 Rating (155 Votes )
There are 3 Steps involved in it
Analyze Your Answer The sum of the two reactions is So it is fairly easy to see th... View full answer

Get step-by-step solutions from verified subject matter experts


