Iodine is not very soluble in water, but it dissolves readily in a solution containing iodide ions
Question:
Iodine is not very soluble in water, but it dissolves readily in a solution containing iodide ions by the following reaction:
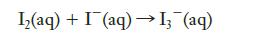
The following graph shows the results of a study of the temperature dependence of ΔG° for this reaction. (The solid line is a best fit to the actual data points.)
Notice that the quantity on the y axis is ΔG°/T, not just ΔG°. Additional data relevant to this reaction are also given in the table that follows the graph.
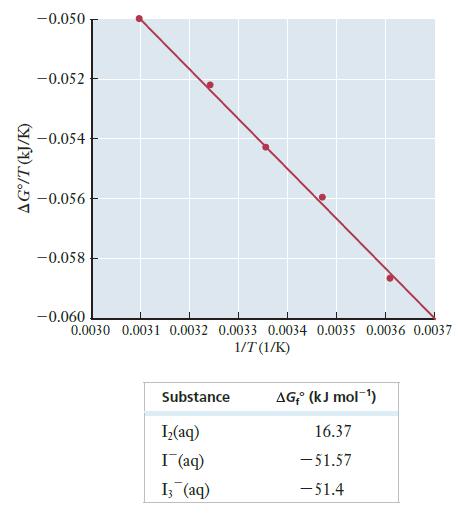
(a) Calculate ΔG° for this reaction at 298 k. (DO NOT read this value off the graph. Use the data given to calculate a more accurate value.)
(b) Determine ΔH° for this reaction. Assume that ΔH° is independent of T. ( You will need to use the graph provided to find ΔH°. It may help if you realize that the graph is a straight line and then try to write an equation for that line.)
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





