Most first aid cold packs are based on the endothermic dissolution of ammonium nitrate in water: A
Question:
Most first aid “cold packs” are based on the endothermic dissolution of ammonium nitrate in water:
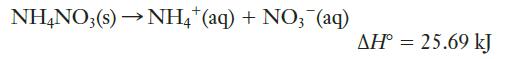
A particular cold pack contains 50.0 g of NH4NO3 and 125.0 g of water. When the pack is squeezed, the NH4NO3 dissolves in the water. If the pack and its contents are initially at 24.0°C, what is the lowest temperature that this bag could reach? (Assume that the ammonium nitrate solution has a specific heat of 4.25 J g-1 K-1, and that the heat capacity of the bag itself is small enough to be neglected.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





