Polymerization reactions are complicated somewhat because they involve very large numbers of molecules. But we can demonstrate
Question:
Polymerization reactions are complicated somewhat because they involve very large numbers of molecules. But we can demonstrate the general features of the thermodynamics of polymerization by considering a much smaller model system. Instead of considering the formation of polyethylene, for example, we can begin with the following reaction in which two ethylene molecules combine with hydrogen to form butane:
![]()
Use data from Table 10.1 to calculate S° for this reaction.
Table 10.1
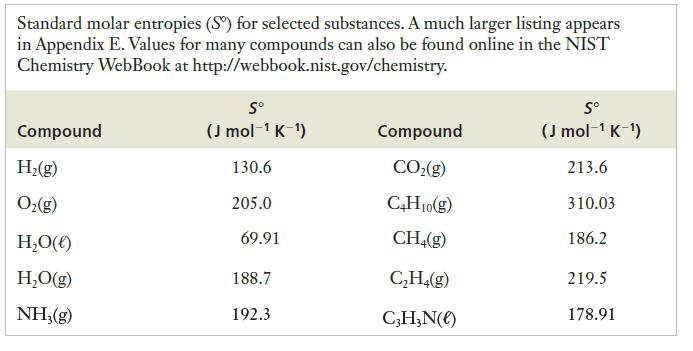
Strategy Any time we are asked to calculate the standard entropy change for a reaction, our first thought should be to look up values for standard molar entropy and use them in Equation 10.3. The two main things we need to be careful about are (1) to watch the state of the substances (in this case all are gases) and (2) to make sure we don’t forget to include the stoichiometric coefficients in our calculations. Unlike heats of formation, the standard molar entropy of an element in its standard state is not zero, so we need to be sure to include everything appearing in the equation.
Equation 10.3
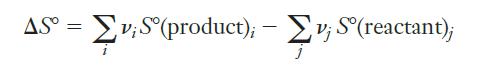
Step by Step Answer:

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme





