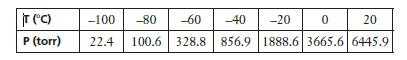
The following data show the vapor pressure of liquid propane as a function of temperature. (a) Plot
Question:
The following data show the vapor pressure of liquid propane as a function of temperature.
(a) Plot a vapor pressure curve for propane and use it to estimate the normal boiling point.
(b) Use your curve to estimate the pressure (in atm) in the propane tank supplying fuel for a gas barbecue grill on a hot summer day when the temperature is 958F.
(c) What implications might your answer to part (b) have for an engineer designing propane storage tanks?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





