Use VSEPR theory to determine the shape of the NOF molecule. Strategy Once again, we start by
Question:
Use VSEPR theory to determine the shape of the NOF molecule.
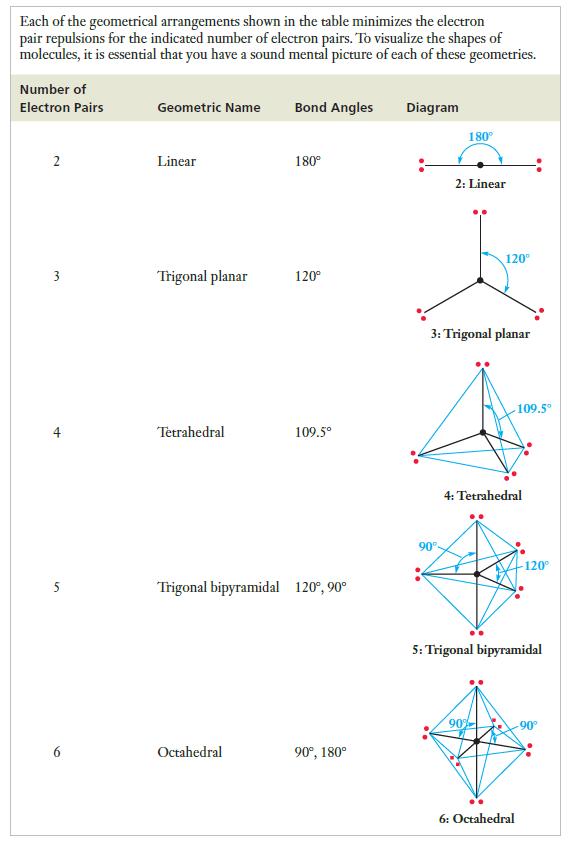
Strategy Once again, we start by drawing the Lewis structure. Then count the number of regions of electron density around the central atom, remembering to count any double or triple bonds as a single region. Determine the spatial arrangement of electron pairs, consulting Table 7.3 as needed. Place any lone pairs in positions where the electron repulsions are minimized and describe the resulting geometric arrangement of the atoms.
Table 7.3
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





