Question: Hydrazine would be expected to adopt a conformation in which the N~H bonds stagger. There are two likely candidates, one with the lone pairs on
Hydrazine would be expected to adopt a conformation in which the N~H bonds stagger. There are two likely candidates, one with the lone pairs on nitrogen anti to each other and the other with the lone pairs gauche:
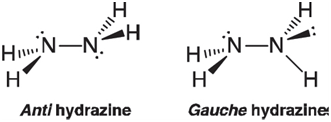
On the basis of the same arguments made in VSEPR theory (electron pairs take up more space than bonds), you might expect that anti hydrazine would be the preferred structure.
a. Obtain energies for the anti and gauche conformers of hydrazine using the HF/6-31G* model. Which is the more stable conformer? Is your result in line with what you expect from VSEPR theory?
b. Measure the energy of the highest occupied molecular orbital (the HOMO) for each of the two hydrazine conformers. This corresponds to the higher-energy (destabilized) combination of electron pairs. Which hydrazine conformer (anti or gauche) has the higher HOMO energy? Is this also the higher-energy conformer? If so, is the difference in HOMO energies comparable to the difference in total energies between the conformers?
HN-N Gauche hydrazine N-N. `H. Anti hydrazine
Step by Step Solution
3.51 Rating (181 Votes )
There are 3 Steps involved in it
a Hydrazine According to HF631G calculations gauche hydrazine is 12 kJmol lower in ... View full answer

Get step-by-step solutions from verified subject matter experts


