Question: Using only the data given below, determine G for the following reaction: NO(g) + O(g) NO(g) (Remember that AG is a state function, just like
Using only the data given below, determine ΔG° for the following reaction:
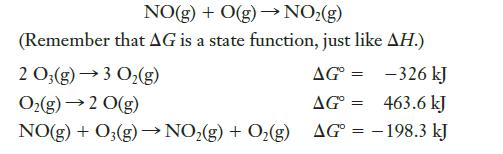
NO(g) + O(g) NO(g) (Remember that AG is a state function, just like AH.) 2 O3(g) 3 O(g) AG= -326 kJ O(g) 2 0(g) AG = 463.6 kJ NO(g) + O(g) NO(g) + O(g) AG = -198.3 kJ
Step by Step Solution
3.51 Rating (151 Votes )
There are 3 Steps involved in it
To determine G for the reaction NOg Og NO2gwe can use the following Hesss Law cycl... View full answer

Get step-by-step solutions from verified subject matter experts


