The graph below shows G as a function of temperature for the synthesis of ammonia from nitrogen
Question:
The graph below shows ΔG° as a function of temperature for the synthesis of ammonia from nitrogen and hydrogen.
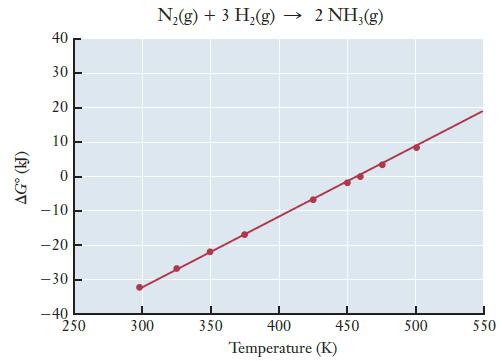
(a) Use the graph to estimate ΔS° for the ammonia synthesis reaction.
(b) Given that the standard free energy change of formation for ammonia (ΔGf°) is –16.50 kJ/mol, estimate ΔH° for the ammonia synthesis reaction.
Transcribed Image Text:
AGⓇ (kJ) 40 30 20 10 0 -10 -20 -30 -40 1 250 300 N₂(g) + 3 H₂(g) → 2 NH3(g) 350 450 400 Temperature (K) 500 550
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
a 2...View the full answer

Answered By

Parvesh Kumar
I am an experienced Mathematics and Statistics tutor with 10 years of experience teaching students and working professionals. I love teaching students who are passionate to learn subjects or wants to understand any mathematics and statistics concept at graduation or master’s level. I have worked with thousands of students in my teaching career. I have helped students deal with difficult topics and subjects like Calculus, Algebra, Discrete Mathematics, Complex analysis, Graph theory, Hypothesis testing, Probability, Statistical Inference and more. After learning from me, students have found Mathematics and Statistics not dull but a fun subject. I can handle almost all curriculum of mathematics. I did B.Sc (mathematics), M.Sc (mathematics), M.Tech (IT) and am also Gate (CS) qualified. I have worked in various college and school and also provided online tutoring to American and Canadian students. I look forward to discussing with you and make learning a meaningful and purposeful
5.00+
4+ Reviews
10+ Question Solved
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:
Students also viewed these Sciences questions
-
Ammonia is produced directly from nitrogen and hydrogen by using the Haber process. The chemical reaction is N2 (g) + 3H2 (g) 2NH3 (g) (a) Use Table 8.4 to estimate the enthalpy change for the...
-
The catalytic reaction A B takes place within a fixed bed containing spherical porous catalyst X22. Figure P15-2B shows the overall rates of reaction at a point in the reactor as a function of...
-
Ammonia is produced by the Haber process, in which nitrogen and hydrogen are reacted directly using an iron mesh impregnated with oxides as a catalyst. For the reaction N2(g) + 3H2(g) 2NH3(g)...
-
A psychologist shows a list of eight activities to a subject in an experiment. How many ways can the subject pick a first, second, and third activity? a. Identify the total number of objects n and...
-
The Anson Manufacturing Company reviewed its year-end inventory and found the following items. Indicate which items should be included in the inventory balance at December 31, 2011. Give your reasons...
-
1. If inflation increases less than expected, the actual unemployment rate will be ________ (above/below) the natural rate. 2. Robert E, Lucas, Jr., explained business cycles by rules of thumb._____...
-
Do the following activities to complete your marketing plan: 1. Draw a simple organizational chart for your organization. 2. Develop a Gantt chart (see Chapter 2) to schedule the key activities...
-
Ethics and pricing. Instyle Interior Designs has been requested to prepare a bid to decorate four model homes for a new development. Winning the bid would be a big boost for sales representative Jim...
-
Using concepts from Appendix B on the accompanying CD with this book on the DDM and this chapter, answer the following questions. (a) Derive an expression for the P/E ratio in terms of the pay out...
-
Suppose that you need to know the heat of formation of cyclohexane, C 6 H 12 , but the tables you have do not provide the value. You have a sample of the chemical. What could you do to determine the...
-
Using only the data given below, determine G for the following reaction: NO(g) + O(g) NO(g) (Remember that AG is a state function, just like AH.) 2 O3(g) 3 O(g) AG= -326 kJ O(g) 2 0(g) AG = 463.6...
-
From the following data ascertain the size of the provision for doubtful debts for each year, stating the entry needed in the respective years statement of comprehensive income. In each case, the...
-
In a year when inventory increased from $100,000 to $140,000, Toffee Co. had sales of $500,000 on which a gross profit of 40% was earned. On average, how many days did it take the company to sell its...
-
Players in the Indian smartphone industry release new models every few months in an attempt to ensure that their products stay on top. This means that once a new model is released, the demand for...
-
Identify an advantage of a public listing from the perspective of the issuer. Ease of raising additional capital due to established market value. Costs of exchange listing. Need to disclose material...
-
Which financial statement would you find Property, Plant and Equipment, Intangibles and/or Natural Resources? explain why.
-
What is the day of the independence of Mexico from Spain?
-
The Canton Corporation operates in four distinct business segments. The segments, along with 2011 information on revenues, assets and net income, are listed below ($ in millions): Required: 1. For...
-
Write a paper by answer the following question: Should Recycling Be Mandatory?
-
Determine the number of permutations of size 3 that can be made from the set {1, 2, 3, 4, 5, 6}. Write down all of the permutations.
-
Determine the numerical values for the following: a. The number of configurations employing all objects in a six-object set. b. The number of configurations employing four objects from a six-object...
-
Radio station call letters consist of four letters (for example, KUOW). a. How many different station call letters are possible using the 26 letters in the English alphabet? b. Stations west of the...
-
(8%) Problem 2: Common static electricity involves charges in the range between nanocoulombs and microcoulombs. 50% Part (a) How many electrons are needed to form a charge of Q = -7 nC? N= sin Cos...
-
Both marketing and marketing communication are changing rapidly. New tools and methods are coming up and especially due t o changes in technology and the consumers themselves. Despite all these...
-
What role does effective leadership play in orchestrating collaborative efforts towards common goals and objectives?

Study smarter with the SolutionInn App


