When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay: The
Question:
When formic acid is heated, it decomposes to hydrogen and carbon dioxide in a first-order decay:
![]()
The rate of reaction is monitored by measuring the total pressure in the reaction container.
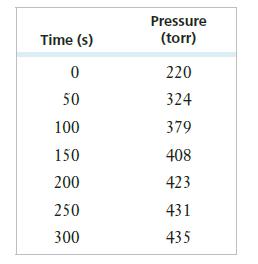
Calculate the rate constant and half-life in seconds for the reaction. At the start of the reaction (time = 0), only formic acid is present. (Find the partial pressure of formic acid using Dalton’s law of partial pressure and the reaction stoichiometry to find PHCOOH at each time.)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry For Engineering Students
ISBN: 9780357026991
4th Edition
Authors: Lawrence S. Brown, Tom Holme
Question Posted:





