A gas sample in a 1.2-L container holds 0.22 mol N 2 and 0.13 mol O 2
Question:
A gas sample in a 1.2-L container holds 0.22 mol N2 and 0.13 mol O2. Calculate the partial pressure of each gas and the total pressure at 50 °C.
Strategy
Use the ideal gas law to calculate the partial pressure of each gas in the container, and sum these two numbers to obtain the total pressure.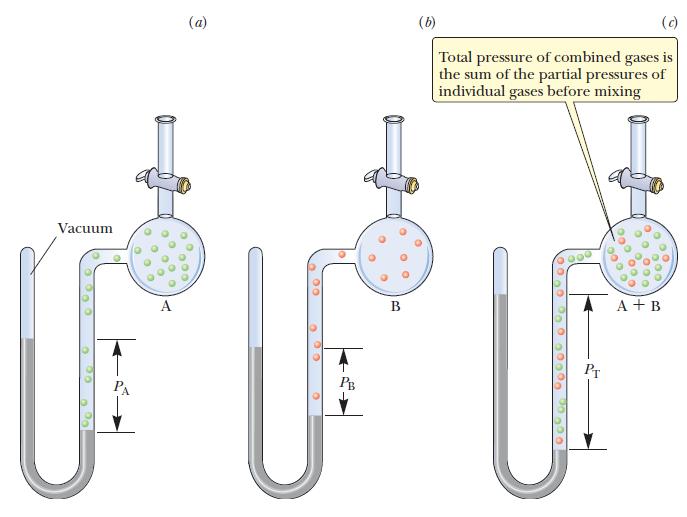
Transcribed Image Text:
Vacuum (a) 200 PB 0 0 D 00 B (b) O (c) Total pressure of combined gases is the sum of the partial pressures of individual gases before mixing 0000 0000 ooo! 0000 00 O 0.000 30 000 A + B
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
Make a table of the information given PN Po PN Poz VN2 12 L Vo 12 L ...View the full answer

Answered By

Ankur Gupta
I have a degree in finance from a well-renowned university and I have been working in the financial industry for over 10 years now. I have a lot of experience in financial management, and I have been teaching financial management courses at the university level for the past 5 years. I am extremely passionate about helping students learn and understand financial management, and I firmly believe that I have the necessary skills and knowledge to effectively tutor students in this subject.
4.80+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
A 12.5-L scuba diving tank contains a heliumoxygen (heliox) mixture made up of 24.2 g of He and 4.32 g of O 2 at 298 K. Calculate the mole fraction and partial pressure of each component in the...
-
Alset Inc. is considering manufacturing and selling high-end electric automobiles for the next four years. It hired two research and development teams. Team #1: This team believes that it would be...
-
A mixture containing 0.765 mol He (g), 0.330 mol Ne (g) and 0.110 mol Ar (g) is confined in a 10.00-L vessel at 25oC. (a) Calculate the partial pressure of each of the gases in the mixture. (b)...
-
Watts and Strogatz proposed a hybrid model that contains typical links of vertices near each other (people know their geographic neighbors), plus some random long-range connection links. Plot the...
-
The classical probability distribution function for a particle in a box of length L is given by P(x) = 1/L. Use this to find (x) and (x2)for a classical particle in such a box.
-
F&s best-selling item is a standard office desk. The desk consists of four legs, one top, one drawer, two side panels, one back panel, and two drawer guides, as shown below. Each side is assembled...
-
How might the attributes of a highly motivated employee change for different job descriptions?
-
Greene Sisters has a DSO of 20 days. The companys average daily sales are $20,000. What is the level of its accounts receivable? Assume there are 365 days in a year.
-
A mortgage of $300,000 is to be repaid over a 25 year period at a fixed annual interest rate of 4.5%. Calculate the monthly repayments. Now suppose the potential purchaser of the mortgage can afford...
-
As part of a circus performance, a man is attempting to throw a dart into an apple which is dropped from an overhead platform. Upon release of the apple, the man has a reflex delay of 215...
-
Why do 1 mol N 2 and 1 mol O 2 both exert the same pressure if placed in the same 20-L container? Is the mass of the gas sample the same in both cases? Explain why it is the same or diff erent, and...
-
Hydrogen, H 2 , and chlorine, Cl 2 , react to form hydrogen chloride, HCl. Calculate the volume of HCl formed by the reaction of 2.34 L H 2 and 3.22 L Cl 2 .
-
The data in the table describe the relationship between altitude and air temperature. a. Write a best-fit equation for f (x) that describes the relationship (altitude in meters, temperature in...
-
An ETFs tracking error, as traditionally reported, indicates to investors: A. whether the ETF is underperforming or outperforming its underlying index. B. the magnitude by which an ETFs returns...
-
Which of the following statements regarding exchange-traded funds (ETFs) is correct? ETFs: A. disclose their holdings on a quarterly basis. B. trade in both primary and secondary markets. C. offer a...
-
To best assess an ETFs performance, which reflects the impact of portfolio rebalancing expenses and other fees, an investor should: A. review daily return differences between the ETF and its...
-
Identify the ethical concerns posed by Dixons actions and conduct. Mason Dixon, CFA, a portfolio manager with Langhorne Advisors (Langhorne), has just completed the request for proposal (RFP) for the...
-
Assuming arbitrage costs are minimal, which of the following is most likely to occur when the share price of an ETF is trading at a premium to its intraday NAV? A. New ETF shares will be created by...
-
Distinguish between the pairs of terms in each of these three classifications of law: a. Public law, private law. b. Civil law, criminal law. c. Felonies, misdemeanors.
-
Solve each problem. Find the coordinates of the points of intersection of the line y = 2 and the circle with center at (4, 5) and radius 4.
-
Would the trial wave function have been a suitable choice for the calculations carried out in Section 21.4? Justify your answer. 3) 48) () %3D 0 < x < a
-
Is (1, 2) = 1s(1) (1)1s(2) (2) + 1s(2) (2)1s(1) (1) an eigenfunction of the operator S z ? If so, what is its eigenvalue M S ?
-
Calculate the angles that a spin angular momentum vector for an individual electron can make with the z axis.
-
If money is worth 5.3%/year compounded monthly, determine the present value of a debt of $3000 due in 48 years with interest at 6.6%/year compounded semi-annually. Determine (within one basis...
-
Develop and analyze an illustrative example of a capital budgeting decision model for a multinational corporation. Assess the outline and results of this illustrative model and make specific...
-
What are the challenges associated with vaccine development, particularly in designing immunogens that elicit protective immune responses while minimizing adverse reactions or potential escape...

Study smarter with the SolutionInn App


