A solution contains 2.00 g of the nonvolatile solute urea (molar mass = 60.06 g/mol) dissolved in
Question:
A solution contains 2.00 g of the nonvolatile solute urea (molar mass = 60.06 g/mol) dissolved in 25.0 g water. Using the data in Table 12.4, calculate the freezing and boiling points of the solution in degrees Celsius.
Table 12.4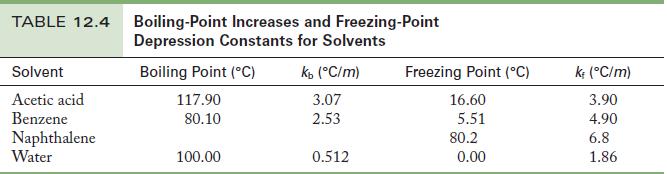
Transcribed Image Text:
TABLE 12.4 Boiling-Point Increases and Freezing-Point Depression Constants for Solvents Solvent Acetic acid Benzene Naphthalene Water Boiling Point (C) 117.90 80.10 100.00 kb (C/m) 3.07 2.53 0.512 Freezing Point (C) 16.60 5.51 80.2 0.00 k; (C/m) 3.90 4.90 6.8 1.86
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
T f ...View the full answer

Answered By

Mary Boke
As an online tutor with over seven years of experience and a PhD in Education, I have had the opportunity to work with a wide range of students from diverse backgrounds. My experience in education has allowed me to develop a deep understanding of how students learn and the various approaches that can be used to facilitate their learning. I believe in creating a positive and inclusive learning environment that encourages students to ask questions and engage with the material. I work closely with my students to understand their individual learning styles, strengths, and challenges to tailor my approach accordingly. I also place a strong emphasis on building strong relationships with my students, which fosters trust and creates a supportive learning environment. Overall, my goal as an online tutor is to help students achieve their academic goals and develop a lifelong love of learning. I believe that education is a transformative experience that has the power to change lives, and I am committed to helping my students realize their full potential.
5.00+
4+ Reviews
22+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.22 m glycerol (C3H8O3) in ethanol, (b) 0.240 mol of naphthalene (C10H8) in 2.45 mol of...
-
Using data from Table 13.3, calculate the freezing and boiling points of each of the following solutions: (a) 0.25 m glucose in ethanol; (b) 20.0 g of decane, C10H22, in 50.0 g CHCl3; (c) 3.50 g NaOH...
-
What is the boiling point of a solution composed of 15.0 g of CHCl 3 and 0.515 g of the nonvolatile solute acenaphthene, C 12 H 10 , a component of coal tar?
-
Your friend has just started a retail clothing store in kamloops. She will be purchasing inventory to make her own clothing (One Style) and she will also be buying ready to sell iteams. She has come...
-
In a balanced three-phase wye-wye system, the load impedance is 10 + j1. The source has phase sequence abc and the line voltage Vab = 220 < 30o V rms. If the load voltage VAN = 120 < 0o V rms,...
-
Explain how an understanding of logistics management could be relevant to your favorite charitable organization.
-
Assume that \(30 \%\) of the laser diodes in a batch of 100 meet the minimum power requirements of a specific customer. If a laser diode is selected randomly, that is, each laser diode is equally...
-
The debate regarding CFLs versus incandescent bulbs (see Problems 25-27) has even more wrinkles. In no particular order: 1. Incandescent bulbs generate a lot more heat than CFLs. 2. CFL prices will...
-
The Sanders Company issued 9.5% bonds, dated January 1, with a face amount of $8,000,000 on January 1, 2024. The bonds mature on December 31, 2033 (10 years). For bonds of similar risk and maturity,...
-
How many grams of water must be added to 4.00 g urea [CO(NH 2 ) 2 ; molar mass = 60.06 g/mol] to produce a 0.250-m solution of the compound?
-
Cyclohexane (C 6 H 12 ) has a vapor pressure of 99.0 torr at 25 C. What is the vapor pressure (in torr) of cyclohexane above a solution of 14.0 g naphthalene (C 10 H 8 ) in 50 g cyclohexane at 25 C?
-
Outline the traditional problems associated with the flat- file model that are resolved by the database model.
-
identify an organization diversification strategy that did not succeed in creating competitive advantage. Explain why you believe it failed. Analyze how, if approached differently, the...
-
address the following: Discuss three factors that may contribute to prison sentences of excessive length and explain how you would correct the problem. Discuss at least three factors that may...
-
If you are familiar with construction, share with the class what you regularly see as the biggest safety challenge. Examples are great, if you have some to share! If you are not familiar with...
-
What was the deviant behavior? What was the punishment, if any? What are the ramifications on sport and society due to this particular situation? Is this behavior a common theme in sports? If so,...
-
1. In Georgia, identify capabilities specifically associated with the fight against terrorism, active shootings, or lone wolf assailants. 2. As a local emergency manager, how can you incorporate...
-
Although all nine of the competitive priorities discussed in this chapter are relevant to a companys success in the marketplace, explain why a company should not necessarily try to excel in all of...
-
1. Use these cost, revenue, and probability estimates along with the decision tree to identify the best decision strategy for Trendy's Pies. 2. Suppose that Trendy is concerned about her probability...
-
Determine the reactions at the supports A and B of the compound beam. Assume there is a pin at C. 18 kN 4 kN/m |C -2 m- -2 m- 6 m
-
Determine the reactions at the supports A, B, D, and F. 2 k/ft Hc F D- -4 ft-4 ft- +- 4 ft-- 4 ft 2 ft -8 ft
-
Determine the reactions on the beam.The support at B can be assumed to be a roller. 2 k/ft 12 ft 12 ft
-
Six runners are entered in a 100-meter dash for which a gold, silver, and bronze medal will be awarded for first, second, and third place finishes, respectively. In how many possible ways can the...
-
A certain message exchange system only enables the use of the 26 uppercase Roman letters, without any spaces, punctuation, or special characters. The letters A through Z take numerical values in...
-
You have now seen trigonometric ratios shown using a) right triangles, b) the Cartesian plane, and c) in graphs of trigonometric functions. Use the equation sin (0) = 1 to make connections between...

Study smarter with the SolutionInn App


