A solution is prepared by dissolving 0.121 g uric acid, C 5 H 3 N 4 O
Question:
A solution is prepared by dissolving 0.121 g uric acid, C5H3N4O3H (molar mass = 168 g/mol), and diluting to make exactly 10 mL of solution. Each uric acid molecule has only one hydrogen ion that dissociates. Conductivity measurements indicate that the acid is 4.2% ionized. Calculate Ka for uric acid, a compound that plays an important role in gout.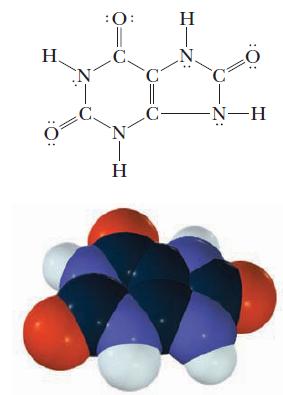
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





