A solution is prepared by dissolving 1.00 g of a nonvolatile solute in 15.0 g acetic acid.
Question:
A solution is prepared by dissolving 1.00 g of a nonvolatile solute in 15.0 g acetic acid. The boiling point of this solution is 120.17 °C . Use the data in Table 12.4 to fi nd the molar mass of the solute.
Strategy
Solve this problem in two stages:
1. Calculate the number of moles of solute in the sample.
2. Calculate the molar mass by dividing the mass of the solute by the number of moles.
From the boiling point of the solution and the information given in Table 12.4 for the solvent, acetic acid, you can calculate the molality.
Table 12.4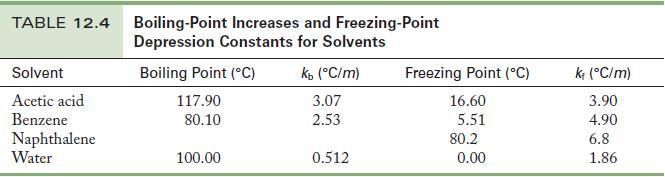
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





