Addition of water to concentrated sulfuric acid is dangerous because it generates enough heat to boil the
Question:
Addition of water to concentrated sulfuric acid is dangerous because it generates enough heat to boil the water, causing it to spatter out of the container. For this reason, chemists remember to add acid to water. In a dilution experiment, we calculate the amount of the more concentrated solution that must be measured out. If we place this concentrated solution in the volumetric flask first, then dilute with water, we violate the caution “Add acid to water.” Describe a safer variation on the method shown in Figure 4.7 that allows the quantitative dilution of concentrated sulfuric acid.
Figure 4.7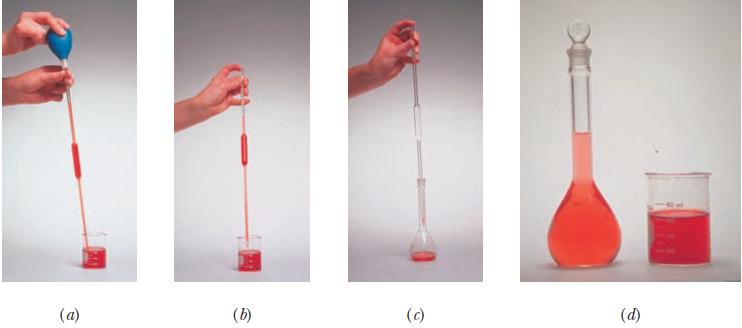
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





