Answer the following questions by using the phase diagram in Exercise 11.43. (a) Sketch the heating curve
Question:
Answer the following questions by using the phase diagram in Exercise 11.43.
(a) Sketch the heating curve that is expected when heat is added to the sample at constant pressure, starting at point B.
(b) Describe what happens if the pressure is lowered at constant temperature, starting at point A.
(c) What does the positive slope of the solid-liquid equilibrium line tell you about this substance?
Exercise 11.43
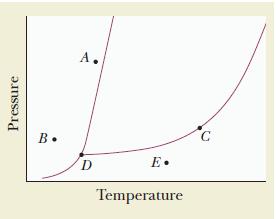
Use the accompanying phase diagram to do the following:
- Label each region of the diagram with the phase that is present.
- Identify the phase or phases present at each of the points A, B, C, D, and E.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





