Balance each of the following redox reactions in acid solution.
Question:
Balance each of the following redox reactions in acid solution.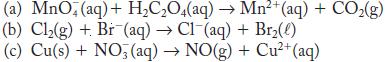
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a Fes 2Agaq 2Ags F...View the full answer

Answered By

Munibah Munir
I've done MS specialization in finance’s have command on accounting and financial management. Forecasting and Financial Statement Analysis is basic field of my specialization. On many firms I have done real base projects in financial management field special forecasting. I have served more than 500 Clients for more than 800 business projects, and I have got a very high repute in providing highly professional and quality services.I have capability of performing extra-ordinarily well in limited time and at reasonable fee. My clients are guaranteed full satisfaction and I make things easy for them. I am capable of handling complex issues in the mentioned areas and never let my clients down.
4.60+
467+ Reviews
648+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Israel We I had been in Israel for two weeks. We were in our church tour bus, late at night, driving through the mountainous desert. The night was black no moon and only a few stars. The only light...
-
(a) Estimate the roof temperature under steady-state conditions. (b) Explore the effect of changes in the absorptivity, emissivity, and convection coefficient on the steady-state temperature.
-
Listed below are speeds (mi/h) measured from southbound traffic on I-280 near Cupertino, California (based on data from SigAlert). dhis simple random sample was obtained at 3:30 p.m. on a weekday....
-
Compare distributed databases to database servers.
-
XYZ is a calendar-year corporation that began business on January 1, 2015. For 2015, it reported the following information in its current year audited income statement. Notes with important tax...
-
The Rando Corporation's stock has a beta of 1.5. If the excess return on the stock market increases by 5%, by approximately how much will the excess return on Rando Corporation's stock increase?
-
Balance each of the following redox reactions in basic solution.
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
Bianca Corp. and Uzma Inc. operate in the same industry. The companies total assets, revenue, and net earnings for the years 2013 2016 are provided below. All amounts are in thousands of dollars....
-
This milestone will help you complete Sections II and III of the final project Directions Develop a report that analyzes one company's approach to multinational expansion. Include financial factors...
-
Jarris Inc. just paid $38,000 in corporate taxes, with its average tax rate at 25%. It spent $24,000 in interest expenses. Jarris Inc.'s cost of goods sold was $190,000; its selling and...
-
Discuss the extent to which you believe the academic literature has been successful in designing early warning systems that signal impending crisis situations and outline the difficulties involved in...
-
Define the Definition and Scope of Credit Analysis, Principles Informing Credit Analysis. (Analyzing quantitative measures: important financial ratios, liquidity, leverage, and profitability to build...
-
A good financial strategy can always be improved. Having knowledge of your organization's finances provides the leverage to make decisions that affect the outlook and goals of the company. This...
-
What is the upper misstatement limit (UML), and how is it determined?
-
Which of the following is NOT a magnetic dipole when viewed from far away? a) A permanent bar magnet. b) Several circular loops of wire closely stacked together with the same current running in each...
-
Devise a block-and-tackle arrangement that amplifies the applied force by a factor of three.
-
Imagine that you are a passenger on the International Space Station. While on the station, you are weightless and so is everything else, including wrenches and furniture. Even though everything...
-
Imagine a skydiver who waits a long time before opening her parachute. For simplicity, assume she moves along a straight line. (a) In what direction is the skydiver moving (what is the direction of...
-
Write paper on critical success factors in eGovernment transformation/ implementation? Harvard Referencing is need at least 5.
-
Can you provide your own example of a proposition of policy using the topic of Cal state Northridge?
-
Describe the difference between referential and table-level integrity and provide examples of related constraints in the proposed schema and how each impacts data integrity. Explain the process and...

Study smarter with the SolutionInn App


