Balance each of the following redox reactions in acid solution.
Question:
Balance each of the following redox reactions in acid solution.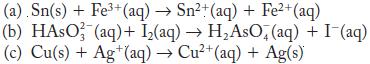
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
The process of balancing redox reactions involves breaking down the reaction into its oxidation and ...View the full answer

Answered By

Aysha Ali
my name is ayesha ali. i have done my matriculation in science topics with a+ . then i got admission in the field of computer science and technology in punjab college, lahore. i have passed my final examination of college with a+ also. after that, i got admission in the biggest university of pakistan which is university of the punjab. i am studying business and information technology in my university. i always stand first in my class. i am very brilliant client. my experts always appreciate my work. my projects are very popular in my university because i always complete my work with extreme devotion. i have a great knowledge about all major science topics. science topics always remain my favorite topics. i am also a home expert. i teach many clients at my home ranging from pre-school level to university level. my clients always show excellent result. i am expert in writing essays, reports, speeches, researches and all type of projects. i also have a vast knowledge about business, marketing, cost accounting and finance. i am also expert in making presentations on powerpoint and microsoft word. if you need any sort of help in any topic, please dont hesitate to consult with me. i will provide you the best work at a very reasonable price. i am quality oriented and i have 5 year experience in the following field.
matriculation in science topics; inter in computer science; bachelors in business and information technology
_embed src=http://www.clocklink.com/clocks/0018-orange.swf?timezone=usa_albany& width=200 height=200 wmode=transparent type=application/x-shockwave-flash_
4.40+
11+ Reviews
14+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Balance each of the following redox reactions in acid solution.
-
Match the phrase that follows with the term (a-e) it describes. estimates the number of units to be manufactured to meet sales and inventory levels integrated set of operating and financing budgets...
-
After being cut from a large single-crystal boule and polished, silicon wafers undergo a high-temperature annealing process. One technique for heating the wafer is to irradiate its top surface using...
-
DNS uses UDP instead of TCP. If a DNS packet is lost, there is no automatic recovery. Does this cause a problem, and if so, how is it solved?
-
A motor, operating on 60 Hz power, has high vibration in the axial direction on the end brackets. The vibration frequency is 7,200 cpm. The motor is only running at 1,180 rpm. What is the excitation...
-
In one stage of an annealing process, 304 stainless steel sheet is taken from 300 K to 1250 K as it passes through an electrically heated oven at a speed of V, = 10 mm/s. The sheet thickness and...
-
Fanning Medical Equipment Company makes a blood pressure measuring kit. Jason McCoy is the production manager. The production department's static budget and actual results for Year 3 follow....
-
Balance each of the following redox reactions in basic solution.
-
Complete and balance each half-reaction in acid solution, and identify it as an oxidation or a reduction.
-
Clorox is the nations leading manufacturer of household liquid bleach (accounting for 49 percent$40 millionof sales annually) and is the only brand sold nationally. Clorox and its next largest...
-
How can online PR help to promote a new product?
-
Name, and briefly explain, four characteristics of an online service that will govern whether a user recommends it.
-
Suggest three measures a company can take to ensure that a customers privacy is not infringed when conducting one-to-one marketing.
-
Explain two applications of dynamic pricing on the Internet.
-
How can different forms of customer insight be used to inform campaign execution?
-
During 2012, Valley Sales, Inc., earned revenues of $500,000 on account. Valley collected $510,000 from customers during the year. Expenses totaled $450,000, and the related cash payments were...
-
Big Jim Company sponsored a picnic for employees and purchased a propane grill equipped with a standard-sized propane tank for the picnic. To make sure there was enough propane for all the cooking...
-
In our discussion of the block and tackle in Figure 3.23, we claimed that when the right end of the string is lifted through a distance L, the body of the pulley is lifted a distance L/2. Give a...
-
An astronaut measures her mass and her weight on Earth and again when she reaches the Moon. Which one of these quantities changes as a result of this trip and which one does not? Explain.
-
One end of a string is tied to the ceiling of an elevator, and the other end is tied to a rock (Fig. Q3.20). The elevator is moving in such a way that the tension in the string is zero. (a) What is...
-
You purchase a 1-year bond from Dell Corporation with a face value of 10,000. When the bond matures in 1 year, how much you can expect to be repaid?
-
In what ways do the complexities of interdepartmental dependencies and cross-functional collaborations hinder the seamless integration of innovative approaches and disrupt the status quo, engendering...
-
On July 1, ABC Company purchased a building for $1,243,000 and uses the 200% declining-balance depreciation method. The estimated residual value of the building is $155,000 and it has an expected...

Study smarter with the SolutionInn App


