Calculate H for the reaction Zn(s) + --0(g) ZnO(s) 2 given the equations AH = ?
Question:
Calculate ΔH for the reaction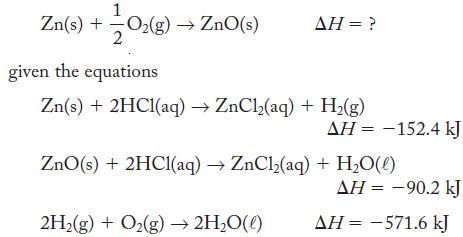
Transcribed Image Text:
Zn(s) + -—-0₂(g) → ZnO(s) 2 given the equations AH = ? Zn(s) + 2HC1(aq) → ZnCl₂ (aq) + H₂(g) 2H₂(g) + O₂(g) → 2H₂O(l) ΔΗ = -152.4 kJ ZnO(s) + 2HC1(aq) →ZnCl₂(aq) + H₂O(0) AH = -90.2 kJ AH = -571.6 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)

Answered By

Isaiah Mutinda
As a graduate with Bs in Maths and Computer Science and having worked as a freelance full stack software developer for 3 years running I believe I have what it takes to conformable tutor and mentor a student to a professional developer also.
5.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculation of H rxn for Part I 0 1. Assume the heat capacity of the final solution is 4.184 J K-1 g-1. Using the final mass of the solution in the calorimeter, calculate q contents from equation...
-
When atoms of the hypothetical element X are placed together, they rapidly undergo reaction to form the X2 molecule: X(g) + X(g) X2(g) a. Would you predict that this reaction is exothermic or...
-
Carbon monoxide and hydrogen can react under different conditions to give different products. One system produces methyl alcohol, CH 3O H (g), when CO and H2 react in the presence of a suitable...
-
What other cost factors might you include in such an economic analysis? The proposed small office building in Example 3-2 has 24,000 net square feet of area heated by a natural gas furnace. The owner...
-
For the conditions of Example 16.8, determine the effect of % recovery of copper over the range of 50100%.
-
Right, or wrong? Say which for each formula and give a brief reason for each answer. a. b. c. [V2x + 1 dx = Vx + x + C 2
-
On 30 June 2025 the following information appeared in the accounting records of Ndung and Mkoka. Balance of Accounts Receivable Control account, \($7450\) Total of schedule of accounts receivable,...
-
Primera Company produces two products and uses a predetermined overhead rate to apply overhead. Primera currently applies overhead using a plantwide rate based on direct labor hours. Consideration is...
-
1. Find the focal length of a convex mirror of radius of curvature 1m. 2. Focal length of a convex mirror is 50 cm. What is its radius of curvature? 3. Radius of curvature of a concave mirror is 25...
-
Jim and Joel have come to you to get an appraisal to lease their property. Using the information below, complete the following questions: Answer the below questions according to the property details...
-
In the process of isolating iron from its ores, carbon monoxide reacts with iron(III) oxide, as described by the following equation: Fe2O3(s) + 3CO(g) 2Fe(s) + 3CO(g) = -24.8 kJ The enthalpy change...
-
Using the thermochemical equations in Exercise 5.67 as needed and in addition Exercise 5.67 Using the following thermochemical equations CH4(g) + 20(g) CO(g) + 2HO(l) CH4(g) + 30(g) 2CO(g) + 2HO(l)...
-
On April 5, Fenning Corporation, a wholesaler of hydraulic lifts, acquired land in exchange for 30,000 shares of $80 par common stock valued at $112 per share. Journalize the entry to record the...
-
The virial equation of state is \[p v=\Re T\left(\mathrm{~b}_{1}+\frac{\mathrm{b}_{2}}{v}+\frac{\mathrm{b}_{3}}{v^{2}}+\ldots . . ight)\] Compare this equation with van der Waals equation of state...
-
A vessel is filled with hydrogen and carbon dioxide in equal parts by volume and the mixture is ignited. If the initial pressure and temperature are \(2 \mathrm{bar}\) and \(60^{\circ} \mathrm{C}\)...
-
A mixture containing hydrogen and oxygen in the ratio of \(2: 1\) by volume is contained in a rigid vessel. This is ignited at \(60{ }^{\circ} \mathrm{C}\) and a pressure of \(1 \mathrm{~atm}(1.013...
-
The exhaust gas from a two-stroke cycle compression-ignition engine is exhausted at an elevated pressure into a large chamber. The gas from the chamber is subsequently expanded in a turbine. If the...
-
The following data refer to an analysis of a dual combustion cycle with a gas having specific heats varying linearly with temperature: The pressure and temperature of the gas at the end of...
-
The duration of a bond that makes an equal payment each year in perpetuity is (1 + yield)/ yield. Prove it.
-
Which of the following streaming TV devices does not involve use of a remote controller? A) Google Chromecast B) Apple TV C) Amazon Fire TV D) Roku
-
Calculate S R for the reaction A g NO 3 (aq) + KCl(aq) A g Cl(s) + KNO 3 (aq).
-
Using the DebyeHckel limiting law, calculate the value of in (a) A 7.2 10 3 m solution of NaBr (b) A 7.50 10 3 m solution of SrCl 2 (c) A 2.25 10 3 m solution of CaHPO 4 . Assume complete...
-
Calculate the mean ionic molality, m , in 0.0750 m solutions of a. Ca(NO 3 ) 2 b. NaOH c. MgSO 4 d. AlCl s .
-
A listed company has hired you to evaluate its recent issue of 2-year TIPS (Treasury Inflation-Protected Securities) with a semi-annual coupon payment clause and requires you to calculate the accrued...
-
Hellriegel et al. define management as 'the process of getting things done, effectively and efficiently, through and with other people'. This makes it seem that management is a simple task, but it is...
-
This task requires reflection to provide insights into the process and quality of personal learning. Your reflection should focus on your actions, motivations and emotions and should analyse what...

Study smarter with the SolutionInn App


