Using the thermochemical equations in Exercise 5.67 as needed and in addition Exercise 5.67 Using the following
Question:
Using the thermochemical equations in Exercise 5.67 as needed and in addition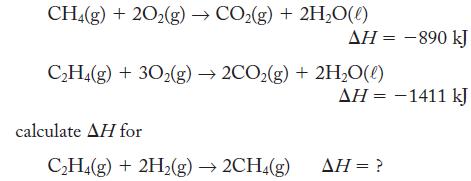
Exercise 5.67
Using the following thermochemical equations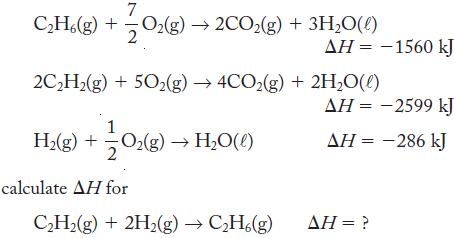
Transcribed Image Text:
CH4(g) + 20₂(g) → CO₂(g) + 2H₂O(l) C₂H4(g) + 30₂(g) →2CO₂(g) + 2H₂O(l) calculate AH for ΔΗ = -890 kJ C₂H4(g) + 2H₂(g) → 2CH4(g) AH = -1411 kJ AH = ?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (2 reviews)
To calculate AH for the reaction C2H4g2H2g 2CH4g we need to use Hesss Law which states that the change in enthalpy for a reaction is the same whether ...View the full answer

Answered By

Hillary Waliaulah
As a tutor, I am that experienced with over 5 years. With this, I am capable of handling a variety of subjects.
5.00+
17+ Reviews
30+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Which of the equations in Exercise 5 represent functions? Data from in Exercise 5 Which equations have a graph that is a vertical parabola? A horizontal parabola? A. y = -x + 20x + 80 C. x + 1 = (y...
-
In Gruber's (1970) study of n = 104 individuals (discussed in Problem 10), the relationship between blood pressure change (SBPSL) and relative weight (RW), controlling for initial blood pressure...
-
The number of counties, divisions, or parishes for each of the 50 states is given below. Use the data to construct a grouped frequency distribution with 6 classes, a histogram, a frequency polygon,...
-
The following items are dropped from an airplane. Rank them in order from lowest terminal speed to highest and justify your ranking. (a) Bowling ball (b) Beach ball (c) Spear or javelin (pointing...
-
For the conditions of Example 16.8, determine the effect on leaching time of particle size over the range of 0.5 mm to 50 mm.
-
For what values of a and b is tan 2x lim x-0 x + a x + sin bx X = 0?
-
Zoe Borrillo, the accountant for Comfy Home Furnishings, was having difficulty completing the trial balance of the businesss general ledger. The balance of the Accounts Receivable Control account in...
-
Sisters Restaurant purchases cheesecakes and offers them as dessert items on its menu. The cheesecakes cost $24 each, and a cake contains 8 pieces. The supplier recently gave notice that the price...
-
9. An arrow 2.5 cm high is placed at a distance of 25 cm from a diverging mirror of focal length 20 cm., Find the nature, position and size of the image formed. 10. The image formed by a convex...
-
On January 1, Year 2, PAT Ltd. acquired 90% of SAT Inc. when SAT's retained earnings were $1,000,000. There was no acquisition differential. PAT accounts for its investment under the cost method. SAT...
-
Calculate H for the reaction Zn(s) + --0(g) ZnO(s) 2 given the equations AH = ? Zn(s) + 2HC1(aq) ZnCl (aq) + H(g) 2H(g) + O(g) 2HO(l) = -152.4 kJ ZnO(s) + 2HC1(aq) ZnCl(aq) + HO(0) AH = -90.2 kJ...
-
Using the following thermochemical equations 7 CH6(g) + O(g)2CO(g) + 3HO(l) 2CH(g) + 50(g) 4CO2(g) +2HO(l) H(g) + 0(g) HO(l) calculate AH for AH = -1560 kJ CH(g) + 2H(g) CH6(g) = -2599 kJ = -286...
-
Current yields are 9 percent on a preferred share that pays a perpetual annual dividend of $6.00. What is the appropriate price of one preferred?
-
A stoichiometric mixture of propane \(\left(\mathrm{C}_{3} \mathrm{H}_{8} ight)\) and air is burned in a constant volume bomb. The conditions just prior to combustion are 10 bar and \(600...
-
Borke Company has a credit balance of $3,000 in Allowance for Doubtful Accounts before adjustment. The total estimated uncollectibles under the percentage-of-receivables basis is $5,800. Prepare the...
-
About 30% of the forests in Japan are managed by the government as national forests. Do you think they should be privatized? Why or why not?
-
(a) An amount of substance equal to 2 kmols of an ideal gas at temperature \(T\) and pressure \(p\) is contained in a compartment. In an adjacent compartment is an amount of substance equal to \(1...
-
The heat of reaction of methane \(\left(\mathrm{CH}_{4} ight)\) is determined in a constant pressure calorimeter by burning the gas as a very weak mixture. The gas flow rate is \(70 \mathrm{~L} /...
-
A bonds credit rating provides a guide to its price. As we write this in Spring 2009, Aaa bonds yield 5.41% and Baa bonds yield 8.47%. If some bad news causes a 10% five-year bond to be unexpectedly...
-
Conduct a VRIO analysis by ranking Husson University (in Maine) business school in terms of the following six dimensions relative to the top three rival schools. If you were the dean with a limited...
-
A weak acid has a dissociation constant of K a = 2.50 10 2 . a. Calculate the degree of dissociation for a 0.093m solution of this acid using the DebyeHckel limiting law. b. Calculate the degree of...
-
Calculate the mean ionic activity of a 0.0350 m Na 3 PO 4 solution for which the mean activity coefficient is 0.685.
-
At 25C, the equilibrium constant for the dissociation of acetic acid, K a , is 1.75 10 5 . Using the DebyeHckel limiting law, calculate the degree of dissociation in 0.150 m and 1.50 m solutions...
-
Looking at the regression model below, how many more units (rounded) are sold when the product is on Feature (feat.a)? (The regression model is statistically significant) Coefficients Standardized...
-
Explain how the answer is found, and also provide the formulas in each cell: Nowjuice, Inc., produces Shakewell fruit juice. A planner has developed an aggregate forecast for demand (in cases) for...
-
Consider that task times of the 7-task problem with precedence diagram given below are normally distributed and the cycle time is equal to 48. Find the solution according to the case P(ST > C) = 0.01...

Study smarter with the SolutionInn App


