Calculate H , S , and G for each of the following reactions at 298 K.
Question:
Calculate ΔH °, ΔS °, and ΔG ° for each of the following reactions at 298 K. State whether the direction of spontaneous reaction is consistent with the sign of the enthalpy change, the entropy change, or both. Use Appendix G for data.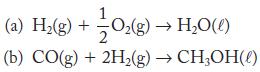
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





