Calculate the empirical formula of a compound extracted from tobacco. Chemical analysis shows that this substance contains
Question:
Calculate the empirical formula of a compound extracted from tobacco. Chemical analysis shows that this substance contains 74.0% carbon , 8.70% hydrogen , and 17.3% nitrogen.
Strategy
The empirical formula is calculated as in Example 3.14 using the percentages as grams in a 100-g sample.
Example 3.14
Analysis of a 0.330-g sample of a compound shows that it contains 0.226 g chromium and 0.104 g oxygen. What is the empirical formula?
Strategy
The strategy is outlined in the following diagram.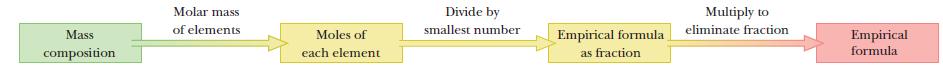
The mass composition values are converted to moles using periodic table information and the molar values converted to whole numbers.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





