Calculate the rms speed in meters per second of argon atoms at 27 C. Strategy Use Equation
Question:
Calculate the rms speed in meters per second of argon atoms at 27 °C.
Strategy
Use Equation 6.6, remembering to use the proper values and units for R, to convert the molar mass into units of kilograms per mole (kg/mol).
Equation 6.6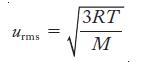
Transcribed Image Text:
Urms 3RT M
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 0% (1 review)
The molar mass of argon 3995 gmol in the proper units i...View the full answer

Answered By

ANDREW KIPRUTO
Academic Writing Expert
I have over 7 years of research and application experience. I am trained and licensed to provide expertise in IT information, computer sciences related topics and other units like chemistry, Business, law, biology, biochemistry, and genetics. I'm a network and IT admin with +8 years of experience in all kind of environments.
I can help you in the following areas:
Networking
- Ethernet, Wireless Airmax and 802.11, fiber networks on GPON/GEPON and WDM
- Protocols and IP Services: VLANs, LACP, ACLs, VPNs, OSPF, BGP, RADIUS, PPPoE, DNS, Proxies, SNMP
- Vendors: MikroTik, Ubiquiti, Cisco, Juniper, HP, Dell, DrayTek, SMC, Zyxel, Furukawa Electric, and many more
- Monitoring Systems: PRTG, Zabbix, Whatsup Gold, TheDude, RRDtoo
Always available for new projects! Contact me for any inquiries
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Calculate the rms speed of helium atoms near the surface of the Sun at a temperature of about 6000K.
-
The pedestrian walkway on the Golden Gate Bridge is about 75 m above the water below. This bridge is (unfortunately) a popular spot for some unhappy people, who attempt to jump off. Ignore air drag...
-
We can measure how good a center Kevin Bacon is by computing each performer's Hollywood number or average path length. The Hollywood number of Kevin Bacon is the average Bacon number of all the...
-
The wave functions for a particle of mass m in a one-dimensional box of length L centered at the origin (so that the ends are at x = ?L/2) are given by Calculate and for the ground state. n= 1, 3, 5,...
-
East Coast Airlines operates a hub at the Pittsburgh International Airport. During the summer, the airline schedules seven flights daily from Pittsburgh to Orlando and ten flights daily from Orlando...
-
What are some of the technological issues that arise through the use of technology in the function of selection?
-
Do you believe one party or the other in the proposed transaction would have an advantage if Pettit & Schayes is hired to perform the due diligence and advise both clients on the arrangement?
-
OX | ChatG ChatG U RealEs New ta Inbox M Inbox Week a single Q single P Pea $449?module_item_id=828652 A G VERABLES Chapter 7 Question 7-28 The following are examples of audit procedures: 1. Watch...
-
The following are the financial statements for Nailsea plc for the years ended 30 June 2014 and 2015: Income statement for years ended 30 June Statements of financial position as at 30 June There...
-
Calculate the molar mass of a gas if equal volumes of oxygen gas and the unknown gas take 3.25 and 8.41 minutes, respectively, to effuse through a small hole under conditions of constant pressure and...
-
Discuss the origin of gas pressure in terms of the kinetic molecular theory.
-
The article Occurrence and Distribution of Ammonium in Iowa Groundwater (K. Schilling, Water Environment Research, 2002:177186) describes measurements of ammonium concentrations (in mg/L) at a large...
-
Explain what additional step(s) Wuyan should have taken in the process of setting capital market expectations. Wuyan reports that after repeatedly searching the most recent 10 years of data, she...
-
Write the missing word(s) in the space provided, using the following words: accounts, asset, buying, cash, cash payments, credit, debit, document, expense, goods, journals, liability, owners equity,...
-
From the transactions of A Gregory for the month of July 2022 where perpetual inventory applies, prepare the sales, purchases, cash receipts and cash payments journals (or general journal); post to...
-
Consider the following series of independent situations in which a firm is about to make a strategic decision. Decisions a. Stila Cosmetics is considering introducing an anti-aging facial cream with...
-
Forrester Fashions has monthly credit sales of 20,000 units with an average collection period of 60 days. The company has a per-unit variable cost of $20 and a per-unit sale price of $30. Bad debts...
-
The United States age discrimination law protects individuals beginning at the age of 40. However, this law has specific requirements regarding whether an employer or the employee determines whether...
-
Perform the operation by first converting the numerator and denominator to scientific notation. Write the answer in scientific notation. 7,200,00/0.000009
-
Why is the magnitude of the electron affinity for a given element smaller than the magnitude of the first ionization energy? Na Ne Li Be Element First Ionization N 13.6 24.6 5.4 13.6 9.3 8.3 14.5...
-
Calculate the position of the maximum in the radial distribution function for Li 2+ in its ground state using the wave function in P21.13.
-
The ground-state wave function of Li 2+ is -1/2 (Z/a 0 ) 3/2 e -Zr /a 0 , where Z is the nuclear charge. Calculate the expectation value of the potential energy for Li 2+ .
-
Question 3 Simon Salt and Paul Pepper were traders, sharing profits and losses in the ratio 3: 1 respectively, and it was agreed that their capitals would remain fixed. The Trial Balance below shows...
-
1. As a project manager, you notice that your team members are spending a lot of time writing comprehensive documentation which contributes to a delay in delivering working software. What can you do...
-
Delph Company uses job-order costing with a plantwide predetermined overhead rate based on machine-hours. At the beginning of the year, the company estimated that 57,000 machine-hours would be...

Study smarter with the SolutionInn App


