Consider the equilibrium Strategy Compute n from the chemical equation, then rearrange the relationship Kp = Kc(RT)
Question:
Consider the equilibrium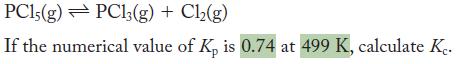
Strategy
Compute Δn from the chemical equation, then rearrange the relationship Kp = Kc(RT)Δn to solve for Kc. Be sure to use 0.08206 L atm/mol K for R and express the temperature in kelvins.
Transcribed Image Text:
PC15(g) PC13(g) + Cl(g) If the numerical value of Kp is 0.74 at 499 K, calculate Ke.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (QA)
First calculate n There are 2 mol of gas on the product ...View the full answer

Answered By

Hassan Imtiaz
The following are details of my Professional Experience. Responsibilities Eight years of demanding teaching experience in the field of finance and business studies at Master’s Level. Completion of the given tasks within given time with quality and efficiency. Marketing professional with practical experience in and solid understanding of a diverse range of management applications, including market analysis, sales and marketing, team building and quality assurance. I have excellent skills to approach deal and sustain corporate clients / customers by demonstrating not only extraordinary communication and interpersonal skills but also high caliber presentation, negotiation and closing skills. Manage and follow up the day-to-day activities. Manage and co-ordinate the inventories. Fulfillment of all the tasks assigned.
The following are details of my Areas of Effectiveness. Finance 1. Corporate Finance 2. Advanced Corporate Finance 3. Management of Financial Institutions 4. International Financial Management 5. Investments 6. Fixed Income 7. Real Estate Investment 8. Entrepreneurial Finance 9. Derivatives 10. Alternative Investments 11. Portfolio Management 12. Financial Statement Analysis And Reporting (US GAAP & IFRS) 13. International Financial Markets 14. Public Finance 15. Personal finance 16. Real estate 17. Financial Planning Quantitative Analysis 1. Time Value Of Money 2. Statistics 3. Probability Distribution 4. Business Statistics 5. Statistical Theory and Methods Economics 1. Principles of Economics 2. Economic Theory 3. Microeconomic Principles 4. Macroeconomic Principles 5. International Monetary Economics 6. Money and Banking 7. Financial Economics 8. Population Economics 9. Behavioral Economics International Business 1. Ethics 2. Business Ethics 3. An introduction to business studies 4. Organization & Management 5. Legal Environment of Business 6. Information Systems in Organizations 7. Operations Management 8. Global Business Policies 9. Industrial Organization 10. Business Strategy 11. Information Management and Technology 12. Company Structure and Organizational Management Accounting & Auditing 1. Financial Accounting 2. Managerial Accounting 3. Accounting for strategy implementation 4. Financial accounting 5. Introduction to bookkeeping and accounting Marketing 1. Marketing Management 2. Professional Development Strategies 3. Business Communications 4. Business planning 5. Commerce & Technology Human resource management 1. General Management 2. Conflict management 3. Leadership 4. Organizational Leadership 5. Supply Chain Management 6. Law 7. Corporate Strategy Creative Writing 1. Analytical Reading & Writing Other Expertise 1. Risk Management 2. Entrepreneurship 3. Management science 4. Organizational behavior 5. Project management 6. Financial Analysis, Research & Companies Valuation 7. And any kind of Excel Queries
4.80+
150+ Reviews
230+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Acetylene, a fuel used in welding torches, is comprised of 92.3% C and 7.7% H by mass. Find the molar mass if 1.10 g of acetylene occupies a volume of 1.00 L at 1.15 atm and 59.50 C. Use 0.08206 L...
-
With respect to charitable contributions, all of the following are true, except: a) Allowing the Girl Scouts to use a building rent-free for one year will not generate a charitable contribution...
-
Fox Erasing has a system of internal control with the following procedures. Match the procedure to the corresponding internal control principle. Procedure Internal Control Principle A. Establish...
-
With reference to Problems 8.5 and 8.6, 9 ft wide by 12 ft high grizzly drifts below the undercut level are first supported with 3/4 in diameter, grade 55 resin bolts 6 ft long (grouted full length)...
-
The worksheet of Millers Landscaping Services follows but is incomplete. Calculate and enter the adjusted account balances in the Adjusted Trial Balancecolumns. MILLER S LANDSCAPING SERVICES...
-
Assume that $y_{i j}$ are i.i.d and follow a normal distribution with variance $\sigma^{2}$. Under the null hypothesis that a factorial effect 0 , a. Show that \[\frac{\bar{y}_{+}-\bar{y}_{-}}{s / 2}...
-
Primus Corp. is planning to convert an existing warehouse into a new plant that will increase its production capacity by 45 percent. The cost of this project will be $7,125,000. It will result in...
-
Suggest a setup to support both file access as well as block access for a SAN with a suitable diagram also give detailed explanation of how this setup can be used.please explain in detail
-
K p for the formation of 2 mol ammonia from nitrogen and hydrogen is 2.8 10 -9 at 298 K. Calculate Kc for N(g) + 3H(g) 2NH3(g)
-
Use the preceding data to calculate Keq at 532 C for 2 (8)H + (8)N (3) HN (9)N : 1.
-
End-of-period adjustments An extract from Birkenhead Ltd's unadjusted trial balance as at 30 June 2026 appears below. Birkenhead Ltd's reporting period ends on 30 June and it uses the perpetual...
-
Create a simple "web-app" that we created using client-side HTML form, come up with our very own (custom-built) JS calculator. One possible "look and feel" is shown below in attached screen-shot (CSS...
-
Under what conditions are short-term contracts preferable to long-term contracts?
-
Discuss how personal insurance needs are similar or not similarto business needs. Choose and describe an insurance product that can be utilized in your personal life and how it can minimize your...
-
Briefly, explain the Uniform Commercial Code. Is it enforceable in all 50 states of the United States?
-
You develop a contract that contains specific language about transportation requirements, and the supplier agrees to it but later claims that it is not acceptable under the UCC. Who in your opinion...
-
Finney Company's condensed income statement is presented below: The following data is compiled relative to Finney's operating segments: Included in the amounts allocated to each segment on the above...
-
In the operation of an automated production line with storage buffers, what does it mean if a buffer is nearly always empty or nearly always full?
-
Express the distance from Chicago to New York City (Table 1.1, page 5) in (a) Miles, (b) Inches, (c) Kilometers, (d) Micrometers. TABLE 1.1 Some Common Lengths and Distances Length or Distance in...
-
A card table has a top surface area of exactly 1 square meter (1 m 2 ). What is its area as measured in (a) Square centimeters and (b) Square millimeters?
-
A bottle has a volume of 1.2 L. (a) What is its volume in cubic centimeters? (b) In cubic meters?
-
Humpty and Dumpty, residents of Miami, Florida, had a nasty incident involving a wall, a fall and a broken egg, in which both parties got hurt. Humpty and Dumpty ended up suing each other in Florida...
-
How could your everyday social media posts prevent you from obtaining employment or destroy your career?
-
How will you store the data for real estate clients? Write brief statement regarding buyer's record keeping.

Study smarter with the SolutionInn App


