Disproportionation is a type of redox reaction in which the same species is simultaneously oxidized and reduced.
Question:
Disproportionation is a type of redox reaction in which the same species is simultaneously oxidized and reduced. One species that undergoes disproportionation is Cu+(aq).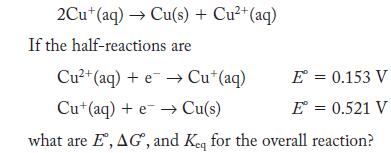
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





