Draw an energy-level diagram based on each of the following thermochemical equations. Label each level with the
Question:
Draw an energy-level diagram based on each of the following thermochemical equations. Label each level with the amounts of substances present, and use an arrow between levels for the given enthalpy change.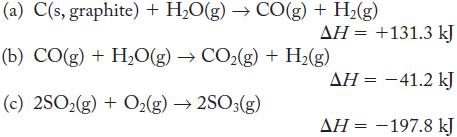
Transcribed Image Text:
(a) C(s, graphite) + HO(g) → CO(g) + H2(g) ΔΗ = +131.3 kJ (b) CO(g) + H,O(g) → CO2(g) + H,(g) (c) 2SO2(g) + O2(g) → 2SO3(g) ΔΗ = -41.2 kJ ΔΗ = -197.8 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a b c ...View the full answer

Answered By

PALASH JHANWAR
I am a Chartered Accountant with AIR 45 in CA - IPCC. I am a Merit Holder ( B.Com ). The following is my educational details.
PLEASE ACCESS MY RESUME FROM THE FOLLOWING LINK: https://drive.google.com/file/d/1hYR1uch-ff6MRC_cDB07K6VqY9kQ3SFL/view?usp=sharing
3.80+
3+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Draw an energy-level diagram based on each of the following thermochemical equations. Label each level with the amounts of substances present, and use an arrow between levels for the given enthalpy...
-
Planning is one of the most important management functions in any business. A front office managers first step in planning should involve determine the departments goals. Planning also includes...
-
Hyten Corporation On June 5, 1998, a meeting was held at Hyten Corporation, between Bill Knapp, Director of Marketing/Sales, and John Rich, director of engineering. The purpose of the meeting was to...
-
Let U = {1, 2, 3, 4,p, 10} A = {1, 3, 5, 7} B = {3, 7, 9, 10} C = {1, 7, 10} Determine the following. A - B
-
Paper is being dried with recirculating air in a two-stage drying system operating at 1 atm. The air enters the first dryer at 180"F, where the air is adiabatically saturated with moisture. The air...
-
Let (x) = px 2 + qx + r be a quadratic function defined on a closed interval [a, b]. Show that there is exactly one point c in (a, b) at which satisfies the conclusion of the Mean Value Theorem.
-
For the accounts of her business Rebecca Goumas uses sales, purchases, cash receipts, cash payments and general journals along with subsidiary ledgers for accounts receivable and accounts payable....
-
Figure shows the BOMs for two products. A and B. Table shows the MPS quantity start date for each one. Table contains data from inventory records for items C, D, and E. There are no safety stock...
-
You are given an undirected graph consisting of n vertices and m edges. It is guaranteed that the given graph is connected (i. e. it is possible to reach any vertex from any other vertex) and there...
-
The whistleblowing aspects of this case were first discussed in the text. What follows is a more comprehensive discussion of accounting and auditing issues. In 2005, Tony Menendez, a former Ernst &...
-
A 0.470-g sample of magnesium reacts with 200 g dilute HCl in a coffee-cup calorimeter to form MgCl 2 (aq) and H 2 (g). Th e temperature increases by 10.9 C as the magnesium reacts. Assume that the...
-
Dissolving 6.00 g CaCl 2 in 300 mL of water causes the temperature of the solution to increase by 3.43 C. Assume that the specific heat of the solution is 4.18 J/g K and its mass is 306 g. (a) ...
-
Winona contracted with XtremeCast, a broadcast media firm, to cohost an Internet-streaming sports program. Winona and XtremeCast signed a new contract for each episode. In each contract, Winona...
-
A cylinder contains \(1 \mathrm{~kg}\) carbon dioxide, and this is compressed adiabatically. Show the pressure, temperature and specific volume are related by the equation \[\frac{1-\alpha}{\alpha}...
-
A gas engine is run on a chemically correct mixture of methane \(\left(\mathrm{CH}_{4} ight)\) and air. The compression ratio of the engine is 10:1, and the trapped temperature and pressure at inlet...
-
A jet engine burns a weak mixture \((\phi=0.32)\) of octane \(\left(\mathrm{C}_{8} \mathrm{H}_{18} ight)\) and air. The air enters the combustion chamber from the compressor at \(10 \mathrm{bar}\)...
-
A gas engine with a volumetric compression ratio of 10:1 is run on a weak mixture of methane \(\left(\mathrm{CH}_{4} ight)\) and air, with an equivalence ratio, \(\phi=0.9\). If the initial...
-
The following exercise helps clarify the difference between statements of fact and statements of inference. Place a familiar item (such as a coffee cup, a chair, or a ballpoint pen) in front of the...
-
Look again at Table. Suppose the spot interest rates change to the following downward-sloping term structure: r1 = 4.6%, r2 = 4.4%, r3 = 4.2% and r4 = 4.0%. Recalculate discount factors, bond prices,...
-
The following information is for Montreal Gloves Inc. for the year 2020: Manufacturing costs Number of gloves manufactured Beginning inventory $ 3,016,700 311,000 pairs 0 pairs Sales in 2020 were...
-
Calculate the solubility of CaCO 3 (K sp = 3.4 10 -9 ) a. In pure H 2 O. b. In an aqueous solution with I = 0.0250 mol kg 1 . For part (a), do an iterative calculation of and the solubility until...
-
Calculate the probability of finding an ion at a distance greater than 1/ from the central ion.
-
Express in terms of + and for a. SrSO 4 b. MgBr 2 c. K 3 PO 4 d. Ca(NO 3 ) 2 . Assume complete dissociation.
-
In two pages, In order for products to be successful in global markets, considerable research must be conducted to ensure the product will meet the needs of locals. Marketers must modify the 4P's to...
-
On January 1, 2019, Jerome Company purchased nontrading equity investments which are irrevocably designated at FVOCI Purchase Transaction price Market value December 31, 2019 cost Security A Security...
-
Identify the revision of the sentence that removes fillers without changing the meaning of the sentence. Isabella was looking for the gross income total in the report when she said she was absolutely...

Study smarter with the SolutionInn App


