Give the notation for each of the following subshells that is an allowed combination of quantum numbers.
Question:
Give the notation for each of the following subshells that is an allowed combination of quantum numbers. If it is not an allowed combination, explain why.![]()
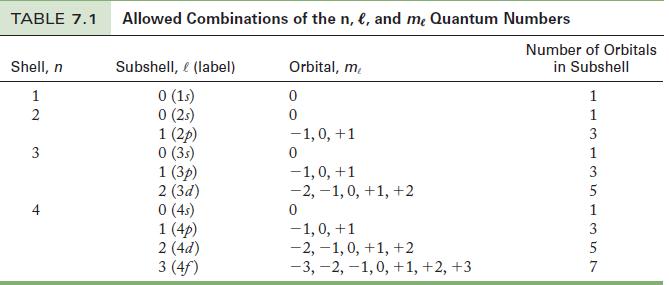
Strategy
Apply the rules for the possible values of n and ℓ.
Transcribed Image Text:
(a) n = 2, l = 0 (b) n = 1, l = 1 (c) n = 4, l = 2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (1 review)
a n 2 0 is an allowed subshell We use the letters ...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Give the notation for each of the following orbitals that is an allowed combination of quantum numbers. If it is not an allowed combination, explain why. Strategy Remember that goes from 0 to (n -...
-
Give the notation for each of the following orbitals that is an allowed combination. If it is not an allowed combination, explain why. (a) n = 2, l = 1, me = 0 (b) n = 5, l = 3, me = -3
-
The following table gives the interiorangle measures of regular polygons with a given number of sides. Number of sides 3 4 5 6 7 8 9 10 11 12 Interiorangle measure 60 90 108 120 128.6 135 140 144...
-
When my niece Michelle told me she had learned to read, she was bursting with pride. How might reflecting on this accomplishment affect how Michelle thinks about herself?
-
Repeat Problem 82 with the gas following path ADC. P, atm 4.0 1.0 4.01 20.0 V, L.
-
List the mistakes that managers commonly make. What distinguishes an arriver from a derailer?
-
Would the combination of a high current ratio and a low inventory turnover ratio suggest a firm is maintaining too high an inventory level? Why or why not?
-
Prior to the start of fiscal 2013, managers of MultiTech hosted a web conference for its shareholders, financial analysts, and members of the financial press. During the conference, the CEO and CFO...
-
Did Wendy's Twitter campaign increase the company's sales and profits? Use the customer journey to speculate about why and how it might or might not have an effect. (660 words max)
-
There is a database that contains a website's traffic data over a period of 30 days. The first table contains users' information including the user type (user, crawler, admin). The second table...
-
Assume that the Earth has warmed by 5K since the last ice age, and the change in radiative forcing over that time was + 6:7 W/m 2 . On this basis, calculate the climate sensitivity. (a) Express the...
-
What is the electron configuration of Zr, whose atomic number is 40?
-
A call center in India used by U.S. and U.K. credit card holders has a capacity of 1,400,000 calls annually. The fixed cost of the center is $775,000 with an average variable cost of $1 and revenue...
-
According to a 2018 article in the Wall Street Journal, proposed tariffs on imported steel could cause the price to consumers of new cars to increase by as much as $300. Use a graph to illustrate the...
-
What is the primary difference between strategic and operating plans?
-
In what sense are you powered by solar energy?
-
When thermal energy is transferred from a hot object to a cold object, the overall entropy A. Decreases B. Increases. C. Does not change.
-
Immediately after the collision, the momentum of the club + ball system will be A. Less than before the collision. B. The same as before the collision. C. Greater than before the collision.
-
Contrast the common-law family with the socialist-law family.
-
Suppose Green Network Energy needs to raise money to finance its new manufacturing facility, but their CFO does not think the company is financially capable of making the periodic interest payments...
-
Propose a synthesis for the following transformation (be sure to count the carbon atoms): Br
-
Compound A is an amine that does not possess a chirality center. Compound A was treated with excess methyl iodide and then heated in the presence of aqueous silver oxide to produce an alkene. The...
-
Phencyclidine (PCP) was originally developed as an anesthetic for animals, but it has since become an illegal street drug because it is a powerful hallucinogen. Treatment of PCP with excess methyl...
-
Tim owns an apartment complex. The lease agreement requires new tenants to pay the first month's rent ( $ 5 , 0 0 0 ) as well as the last month s rent ( $ 5 , 2 2 2 ) , a $ 9 , 0 0 0 security deposit...
-
If you were telling someone about the ways in which automated bidding can improve their campaign management process efficiency, Describe three automated bidding benefits that help them meet their...
-
Table salt is being fed to a vibrating screen at the rate of 3000 kg/hr.The desired product is the 48/65 mesh fraction.A -48 + 65-mesh screen are therefore used, the feed being introduced on the 48...

Study smarter with the SolutionInn App


