Give the notation for each of the following orbitals that is an allowed combination of quantum numbers.
Question:
Give the notation for each of the following orbitals that is an allowed combination of quantum numbers. If it is not an allowed combination, explain why.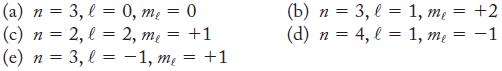
Strategy
Remember that ℓ goes from 0 to (n - 1) and mℓ ranges from -ℓ to ℓ.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





