Question: Identify the missing particles by balancing the mass and atomic numbers in each of the following nuclear decay equations.
Identify the missing particles by balancing the mass and atomic numbers in each of the following nuclear decay equations.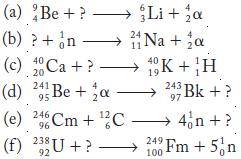
Step by Step Solution
★★★★★
3.36 Rating (159 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

Be Li H The missing particle is an particle He nucleus which has a mass of 4 and an a... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


