If nitric acid were sufficiently heated, it can be decomposed into dinitrogen pentoxide and water vapor: (a)
Question:
If nitric acid were sufficiently heated, it can be decomposed into dinitrogen pentoxide and water vapor: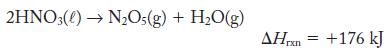
(a) Calculate the enthalpy change that accompanies the reaction of 1.00 kg![]()
(b) Is heat absorbed or released during the course of the reaction?
Transcribed Image Text:
2HNO3()→ N₂O(g) + H₂O(g) AHrxn = +176 kJ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
To calculate the enthalpy change AH for the reaction and determine whether heat is absorbed or r...View the full answer

Answered By

Pakala Ashok Kumar
• Business reports writing and consultancy.
• Proposal development and research writing.
• Timely and accurate submissions of research work
? Excellent computer skills
• MS word
• Excel
• Power-point
• Access
• Internet
• MS publisher
? Fluent spoken and written English language
? Proficient report writing skills.
? Team worker with the ability to inspire a team.
? Ability to follow procedures and meet deadlines at work.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
One step in the manufacturing of sulfuric acid is the conversion of SO 2 (g) to SO 3 (g). The thermochemical equation for this process is The second step combines the SO 3 with H 2 O to make H 2 SO...
-
The heat of solution of ammonia in water at 1 atm is (a) Calculate the enthalpy change that accompanies the dissolution of 200 mol of NH 3 in 400 mol of water at 25C and 1 atm. (b) If you actually...
-
The standard heat of the combustion reaction of liquid n-hexane to form CO 2 (g) and H 2 O(l), with all reactants and products at 77F and 1 atm, is H r = 1:791 10 6 Btu. The heat of vaporization of...
-
A load must be suspended 6 m below a high ceiling using cables attached to two supports that are 2 m apart (see figure). How far below the ceiling (x in the figure) should the cables be joined to...
-
A desublimation unit of the heat-exchanger type is to be sized for the recovery of 200 kg/h of benzoic acid (BA) from a gas stream containing 0.8 mol% BA and 99.2 mol% N 2 . The gas enters the unit...
-
Use Newtons method to find the two real solutions of the equation x 4 - 2x 3 - x 2 - 2x + 2 = 0.
-
All of the following would be considered a company with high operating leverage, except a(n) a. airline company. b. t-shirt kiosk in a mall. c. hotel chain. d. amusement park.
-
Dr. Mark Greenberg practices dentistry in Topeka, Kansas. Greenberg tries hard to schedule appointments so that patients do not have to wait beyond their appointment time. His October 20 schedule is...
-
(08 Marks) In the Fig.1(c), compare the output of the network, if the activation function is a sigmoid 1 function y=- and (X1, X2) (1, 1). 1+e 1+1 AYY Y YC.) B YC. ye D 46 (06 Marks)
-
Cheek Products, Inc. (CPI) was founded 53 years ago by Joe Cheek and originally sold snack foods such as potato chips and pretzels. Through acquisitions, the company has grown into a conglomerate...
-
The thermite reaction produces a large quantity of heat, enough to melt the iron metal that is a product of the reaction: What is the enthalpy change if 50.0 g Al reacts with excess iron(III) oxide?...
-
When lightning strikes, the energy can force atmospheric nitrogen and oxygen to react to make NO: (a) Is this reaction endothermic or exothermic? (b) What quantities of reactants and products are...
-
Explain why this carbocation is considerably more stable than this structure would suggest: H +C-0-CH, H
-
Kidnapping refers to the unlawful taking and carrying away of another person with the intent to deprive that person of his or her liberty. False imprisonment has been defined as forcing a person to...
-
Battery is unlawful touching, typically with injury. Though the actus reus of battery usually requires injury, not all states impose this requirement. A threatened battery assault occurs when one...
-
You have six identical particles in a box divided into four quadrants. For a certain experiment, you need to have a certain number of particles in the upper left quadrant. You observe that in a given...
-
Four distinguishable particles move freely in a room divided into octants (there are no actual partitions). Let the basic states be given by specifying the octant in which each particle is located....
-
Three particles are released into a box that can be thought of as a set of four identical quadrants. How many basic states are possible if the particles are \((a)\) indistinguishable from one another...
-
Refer back to Sections 2.22.4. If the rate of interest is 8% rather than 10%, how much would you need to set aside to provide each of the following? a. $1 billion at the end of each year in...
-
The Strahler Stream Order System ranks streams based on the number of tributaries that have merged. It is a top-down system where rivers of the first order are the headwaters (aka outermost...
-
Using the root mean square speed, v rms = (v 2 ) 1/2 = 3k B T/m, calculate the gas temperatures of He and Ar for which = 0.25 nm, a typical value needed to resolve diffraction from the surface of a...
-
Electrons have been used to determine molecular structure by diffraction. Calculate the speed and kinetic energy of an electron for which the wavelength is equal to a typical bond length, namely,...
-
For a monatomic gas, one measure of the average speed of the atoms is the root mean square speed, v rms = (v 2 ) 1/2 = 3k B T/m, in which m is the molecular mass and k B is the Boltzmann constant....
-
As a manager of an established organization, how would you manage diversity effectively in your workplace? Give adequate explanation.
-
Do you think that Watson and Crick deserve all the credit for DNA's structure? Explain why or why not.
-
How many people must attend the third show so that the average attendance per show is 3000? Theater Attendance 4200 3500 2920 2800 2580 2100 1400 700 1 2 3 Show Equation: - 3000 people need to attend...

Study smarter with the SolutionInn App


