In each part, a set of quantum numbers is given. If the set is an allowed combination
Question:
In each part, a set of quantum numbers is given. If the set is an allowed combination of n, ℓ, mℓ, and ms, give the subshell to which this wave function belongs (1s, 2s, 2p, and so on). If the combination of quantum numbers is not allowed, state why.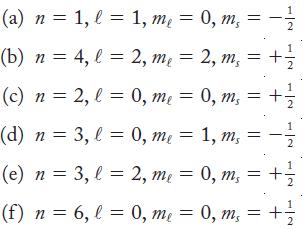
Transcribed Image Text:
(a) n = 1, l = 1, me = 0, m₁ = (b) n = 4, l = 2, me = 2, m₁ = (c) n = 2, l = 0, me = (d) n = 3,l = 0, me = (e) n = (f) n = 3, l = 2, me = 1/2 + =1/2 0, ms = +₁ 1, m, = -1/ 2 0, m₁ = + 1/2 x2 6, 6₂ l = 0, me = 0, m₁ = + 1/2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a n 1 l 1 ml 0 ms 12 Not allowed The value of l cannot be equal to n It mus...View the full answer

Answered By

Manu Sharma
I m post graduate in zoology.i run my own science coatching classes.i have master diploma in laboratory technology with 5 years experience of a endocrine laboratory as a technician.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
In each part, a set of quantum numbers is given. If the set is an allowed combination of n, , m , and m s , give the subshell to which this wave function belongs (1s, 2s, 2p, and so on). If the...
-
assume that in the network shown in Figure 4.1 two parallel TCP transmissions are performed. TCP1 is a transmission between Source A and Sink A that uses TCP Tahoe. TCP2 is a transmission between...
-
The following information provides the amount of cost incurred in March for the cost items indicated. During March, 7,600 units of the firm's single product were manufactured. Raw materials Factory...
-
George and Weezy received $29,100 of Social Security benefits this year ($11,000 for George: $18,100 for Weezy). They also received $4,800 of Interest from jointly owned City of Ranburne Bonds and...
-
A 900-N boy sits on top of a ladder of negligible weight that rests on a frictionless floor as in Figure. There is a cross brace halfway up the ladder. The angle at the apex is q = 30 o . (a) What is...
-
What is motivation? Explain the three key elements of motivation.
-
Consider the patient satisfaction data in Table B.17. Fit a regression model to the satisfaction response using age and severity as the predictors. Perform an influence analysis of the date and...
-
Absorption costing and production volume variance -- alternative capacity bases Earths Best Light (EBL), a producer of energy-efficient light bulbs, expects that demand will increase markedly over...
-
Leonardo, who is married but files separately, earns $ 8 5 , 0 0 0 of taxable income. He also has $ 1 7 , 0 0 0 in city of Tulsa bonds. His wife, Theresa, earns $ 5 2 , 0 0 0 of taxable income. If...
-
An operations manager is trying to determine a production plan for the next week. There are three products (say, P, Q, and Q) to produce using four machines (say, A and B, C, and D). Each of the four...
-
(a) How many subshells are present in the n = 3 shell? (b) How many orbitals are in the 4p subshell? (c) What is the maximum value of that is allowed in the shell with n = 4? (d) What are the...
-
In each part, sketch the contour surface for the orbital described. (a) n = 2, l = 0 (b) 3px (c) 4dxy (d) n = 2, l = 1 (e) n = 1, l = 0
-
Customers arrive at teller windows at a bank at the rate of 12 per hour. A teller is able to process 15 customers per hour on average. If the bank has a goal that customer wait time should be no more...
-
Prove that the intersection of convex sets is a convex set.
-
Youve probably been a victim of stereotyping by others, undoubtedly more often than youd like. At the same time, you may have engaged in stereotyping others, even if youre not especially proud of it....
-
Understanding and using the competitive advantage pyramid. 1. Choose a company you would like to work for. Using the competitive advantage pyramid, analyze the strength (or weakness) of competitive...
-
The electric field produces a potential difference. If you place one electrode \(10 \mathrm{~m}\) below the surface of the water, you will measure the greatest potential difference if you place the...
-
Extreme SpA is a newly established entity. It was set up by an entrepreneur who is generally interested in the business of providing engineering and operational support services to aircraft...
-
A restaurant manager is interested in taking a more statistical approach to predicting customer load. She begins the process by gathering data. One of the restaurant hosts or hostesses is assigned to...
-
Differentiate the following terms/concepts: a. Personality types and money attitudes b. Planners and avoiders c. Moderating and adapting to biases d. "Perfectible judges" and "incorrigible judges"
-
What properties of atomic and molecular systems could you imagine describing using probability distributions?
-
What is the difference between average and root-mean-squared?
-
When is the higher moment of a probability distribution more useful as a benchmark value as opposed to simply using the mean of the distribution?
-
. An engineer is investigating energy loss through windows. The windowpane of interest is 0.350 cm thick, has dimensions of 0.99 m x 1.75 m, and has a thermal conductivity of 0.8 W/(m C). On a given...
-
15. A 1500-kg car making a turn on a dry banked highway ramp with angle of inclination of 10 is experiencing friction. The coefficient of static friction between the tires and the road is 0.40. Draw...
-
If a car is moving at 27 m/s, and begins slowing down at a rate of -3.7 m/s^2, how far will it go before it reaches a stop? (Numerical answer is assumed to be in meters.)

Study smarter with the SolutionInn App


