Question: Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen. A two-step mechanism has been proposed. Identify the rate-limiting step. 2NO(g) + O3(g)
Nitrogen dioxide can react with ozone to form dinitrogen pentoxide and oxygen.![2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1704/3/5/5/041659664e176f1c1704355041072.jpg)
A two-step mechanism has been proposed. Identify the rate-limiting step.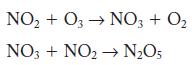
2NO(g) + O3(g) NO5(g) + O(g) rate = /[NO][03]
Step by Step Solution
3.49 Rating (149 Votes )
There are 3 Steps involved in it
To identify the ratelimiting step in the twostep reaction mechanism we look at each step and compare ... View full answer

Get step-by-step solutions from verified subject matter experts


