Nitrogen monoxide reacts with chlorine to form nitrosyl chloride. The figure shows the increase in nitrosyl chloride
Question:
Nitrogen monoxide reacts with chlorine to form nitrosyl chloride.![]()
The figure shows the increase in nitrosyl chloride concentration under appropriate experimental conditions. The concentration of nitrosyl chloride actually starts at zero, although this fact may be difficult to see in the figure.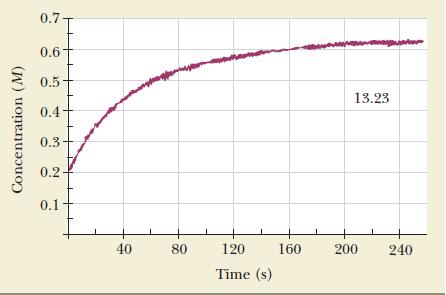
(a) Write an expression for the rate of reaction in terms of a changing concentration.
(b) Calculate the average rate of reaction between 40 and 120 seconds.
(c) Calculate the instantaneous rate of reaction after 80 seconds.
(d) Calculate the instantaneous rate of consumption of chlorine 60 seconds after the start of the reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





