Dinitrogen tetroxide decomposes to nitrogen dioxide under laboratory conditions. (a) Fill in the missing concentrations. (b) Calculate
Question:
Dinitrogen tetroxide decomposes to nitrogen dioxide under laboratory conditions.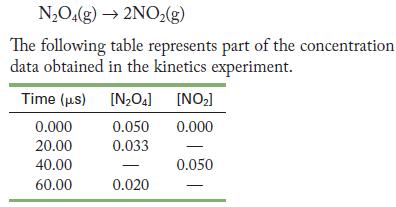
(a) Fill in the missing concentrations.
(b) Calculate the rate of reaction at 30 microseconds.
Transcribed Image Text:
NO4(g) 2NO(g) The following table represents part of the concentration data obtained in the kinetics experiment. Time (us) 0.000 20.00 40.00 60.00 [NO4] [NO] 0.050 0.000 0.033 0.020 0.050 1
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
a NO 0034 ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:
Students also viewed these Sciences questions
-
Dinitrogen tetroxide decomposes to nitrogen dioxide: At 298 K, a reaction vessel initially contains 0.100 atm of N 2 O 4 . When equilibrium is reached, 58% of the N 2 O 4 has decomposed to NO 2 ....
-
Fill in the missing information in the following table. Assume that Portfolio AB is 30 percent invested in StockA. Stock A 11% 37 21 26 13 Stock B Portfolio AB Year 2003 2004 2005 2006 2007 Average...
-
Fill in the missing amounts for a-d. ACCOUNT INFORMATION TypeRevving Account Number 234 98765 90 Biling Date 16 Aug Payment Due Date 1 Sep TRANSACTIONS I DEBITS/ CREDITS (-) 6AUG Moghan's Shop...
-
Southern Stitches is a local casual clothing shop that makes a variety of t-shirt styles. Below is some actual vs. budget information for Southern's t-shirts for the month of May. Assume no...
-
Calculate the three lowest energy levels, together with their degeneracies, for the following systems (assume equal mass distinguishable particles): a. Three non-interacting spin particles in a box...
-
Distinguish between informal venture capital and formal venture capital.
-
With reference to Exercise 4.5, find an expression for the distribution function \(F(x)\) of the random variable. Data From Exercise 4.5 k 4.5 Given that f(x): = is a probability distribution for 2x...
-
What is an opportunity cost? How is this concept used in TVM analysis, and where is it shown on a time line? Is a single number used in all situations? Explain.
-
What is the yield to maturity of a corporate bond that has a coupon rate of 5%, a face value of $1,000, a present value of $574.32, and a time to maturity of 20 years?
-
Under certain conditions, biphenyl, C 12 H 10 , can be produced by the decomposition of cyclohexane, C 6 H 12 : (a) Fill in the missing concentrations. (b) Calculate the rate of reaction at 1.5...
-
Hydrogen iodide forms from hydrogen and iodine: The following figure shows the increase in hydrogen iodide concentration under appropriate experimental conditions. (a) Write an expression for the...
-
In a study designed to test the effectiveness of paracetamol (or acetaminophen) as a treatment for lower back pain, 1643 patients were randomly assigned to one of three groups: (1) The 547 subjects...
-
What do you believe are the top five characteristics of a good leader? What are the top five for a good manager? Discuss the similarities and differences between a manager and a leader. In what ways...
-
Is leadership of a project different in an Agile environment than in a traditional project environment?
-
I need help with transition words or phrases from this sentence,You can make a difference by donating blood as a good citizen. Give me a smooth flow to Someone in the U. S need blood every two...
-
Appositive Exercise Highlight (Bold) and punctuate the appositives in the following sentences. Not all require punctuation. 1. My son, the policeman, will be visiting us next week. 2. The captain...
-
Describe the systems and technology risk of technological disruptions in an emergency for a town in Massachusetts. Describe its causes and effects and their time frames. Explain how you identified it...
-
You are managing a portfolio of $1 million. Your target duration is 10 years, and you can choose from two bonds: a zero-coupon bond with maturity 5 years, and perpetuity, each currently yielding 5%....
-
The Thomas Corporation was organized on Jan. 1, 2020. On Dec. 31, 2021, the corporation lost most of its inventory in a warehouse fire before the year-end count of inventory was to take place. just...
-
If determine A + A T . 2 3 6 5 9 2 2. -1
-
If determine AA T . -1
-
If determine AA T . 2 8 5]' A -1
-
Part One: Le-Nature's Inc. Case Explain the corporate governance-related responsibilities of the internal roles (accountants) within the case. Explain the corporate governance-related...
-
In Table 2 replace Max capacity in the Units column with the value in Table 1. Calculate Mid point by dividing Max capacity by two. Create Excel formulas and use cell references to calculate revenue...
-
Modern Electronics Company purchases merchandise inventory from several suppliers. On April 1, 2023, Modern Electronics purchased from Speedy Supplies $120,000 of inventory on account. On May 15,...

Study smarter with the SolutionInn App


