Predict which species in each of the following pairs has the higher first ionization energy. (a)
Question:
Predict which species in each of the following pairs has the higher first ionization energy.
(a) Mg or P
(b) B or Cl
(c) K+ or Ca2+
Strategy
Use the ionization energy trends—
(1) Up a group, the ionization energies increase;
(2) Across a row from left to right, ionization energies mainly increase—to determine which has the greater ionization energy. For two species that are isoelectronic, the species with the greater charge has the higher ionization energy. Use the small periodic table shown to locate the elements.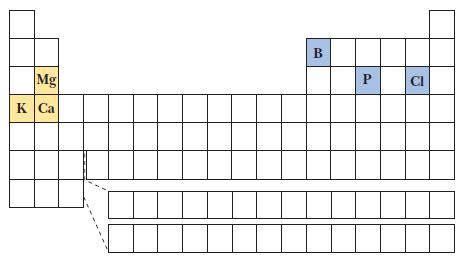
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry Principles And Practice
ISBN: 9780534420123
3rd Edition
Authors: Daniel L. Reger, Scott R. Goode, David W. Ball
Question Posted:





